Microscale gradients of oxygen, hydrogen peroxide, and pH in freshwater cathodic biofilms
- PMID: 23766295
- PMCID: PMC4247834
- DOI: 10.1002/cssc.201300019
Microscale gradients of oxygen, hydrogen peroxide, and pH in freshwater cathodic biofilms
Abstract
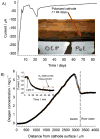
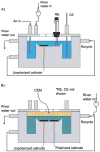
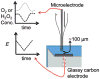
Cathodic reactions in biofilms employed in sediment microbial fuel cells is generally studied in the bulk phase. However, the cathodic biofilms affected by these reactions exist in microscale conditions in the biofilm and near the electrode surface that differ from the bulk phase. Understanding these microscale conditions and relating them to cathodic biofilm performance is critical for better-performing cathodes. The goal of this research was to quantify the variation in oxygen, hydrogen peroxide, and the pH value near polarized surfaces in river water to simulate cathodic biofilms. We used laboratory river-water biofilms and pure culture biofilms of Leptothrix discophora SP-6 as two types of cathodic biofilms. Microelectrodes were used to quantify oxygen concentration, hydrogen peroxide concentration, and the pH value near the cathodes. We observed the correlation between cathodic current generation, oxygen consumption, and hydrogen peroxide accumulation. We found that the 2 e(-) pathway for oxygen reduction is the dominant pathway as opposed to the previously accepted 4 e(-) pathway quantified from bulk-phase data. Biofouling of initially non-polarized cathodes by oxygen scavengers reduced cathode performance. Continuously polarized cathodes could sustain a higher cathodic current longer despite contamination. The surface pH reached a value of 8.8 when a current of only -30 μA was passed through a polarized cathode, demonstrating that the pH value could also contribute to preventing biofouling. Over time, oxygen-producing cathodic biofilms (Leptothrix discophora SP-6) colonized on polarized cathodes, which decreased the overpotential for oxygen reduction and resulted in a large cathodic current attributed to manganese reduction. However, the cathodic current was not sustainable.
Keywords: cyclic voltammetry; electrochemistry; electron transfer; fuel cells; oxygen.
Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures






References
-
- Menicucci J, Beyenal H, Marsili E, Veluchamy RA, Demir G, Lewan-dowski Z. Environ Sci Technol. 2006;40:1062–1068. - PubMed
-
- Bergel A, Feron D, Mollica A. Electrochem Commun. 2005;7:900–904.
- Cao XX, Huang X, Liang P, Boon N, Fan MZ, Zhang L, Zhang XY. Energy Environ Sci. 2009;2:498–501.
- Liang P, Fan MZ, Cao XX, Huang X. J Chem Technol Biotechnol. 2009;84:794–799.
- Rabaey K, Read ST, Clauwaert P, Freguia S, Bond PL, Blackall LL, Keller J. Isme J. 2008;2:519–527. - PubMed
- Ter Heijne A, Strik DPBTB, Hamelers HVM, Buisman CJN. Environ Sci Technol. 2010;44:7151–7156. - PubMed
- Zhang JN, Zhao QL, Aelterman P, You SJ, Jiang JQ. Biotechnol Lett. 2008;30:1771–1776. - PubMed
-
- Rhoads A, Beyenal H, Lewandowski Z. Environ Sci Technol. 2005;39:4666–4671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

