Kupffer cells potentiate liver sinusoidal endothelial cell injury in sepsis by ligating programmed cell death ligand-1
- PMID: 23766529
- PMCID: PMC3800070
- DOI: 10.1189/jlb.0113051
Kupffer cells potentiate liver sinusoidal endothelial cell injury in sepsis by ligating programmed cell death ligand-1
Abstract
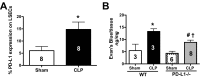
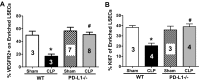
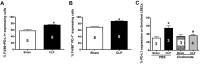
PD-1 and PD-L1 have been reported to provide peripheral tolerance by inhibiting TCR-mediated activation. We have reported that PD-L1-/- animals are protected from sepsis-induced mortality and immune suppression. Whereas studies indicate that LSECs normally express PD-L1, which is also thought to maintain local immune liver tolerance by ligating the receptor PD-1 on T lymphocytes, the role of PD-L1 in the septic liver remains unknown. Thus, we hypothesized initially that PD-L1 expression on LSECs protects them from sepsis-induced injury. We noted that the increased vascular permeability and pSTAT3 protein expression in whole liver from septic animals were attenuated in the absence of PD-L1. Isolated LSECs taken from septic animals, which exhibited increased cell death, declining cell numbers, reduced cellular proliferation, and VEGFR2 expression (an angiogenesis marker), also showed improved cell numbers, proliferation, and percent VEGFR2(+) levels in the absence of PD-L1. We also observed that sepsis induced an increase of liver F4/80(+)PD-1(+)-expressing KCs and increased PD-L1 expression on LSECs. Interestingly, PD-L1 expression levels on LSECs decreased when PD-1(+)-expressing KCs were depleted with clodronate liposomes. Contrary to our original hypothesis, we document here that increased interactions between PD-1(+) KCs and PD-L1(+) LSECs appear to lead to the decline of normal endothelial function-essential to sustain vascular integrity and prevent ALF. Importantly, we uncover an underappreciated pathological aspect of PD-1:PD-L1 ligation during inflammation that is independent of its normal, immune-suppressive activity.
Keywords: acute liver failure; coinhibitory molecules; hepatic endothelium; liver immune tolerance; septic inflammation.
Figures


References
-
- Angus D. C., Linde-Zwirble W. T., Lidicker J., Clermont G., Carcillo J., Pinsky M. R. (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29, 1303–1310 - PubMed
-
- Canabal J. M., Kramer D. J. (2008) Management of sepsis in patients with liver failure. Curr. Opin. Crit. Care 14, 189–197 - PubMed
-
- Aird W. C. (2003) The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 101, 3765–3777 - PubMed
-
- Crispe I. N. (2009) The liver as a lymphoid organ. Annu. Rev. Immunol. 27, 147–163 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

