Isotopically labeled sulfur compounds and synthetic selenium and tellurium analogues to study sulfur metabolism in marine bacteria
- PMID: 23766810
- PMCID: PMC3678758
- DOI: 10.3762/bjoc.9.108
Isotopically labeled sulfur compounds and synthetic selenium and tellurium analogues to study sulfur metabolism in marine bacteria
Abstract
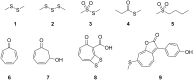
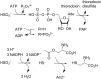
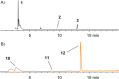
Members of the marine Roseobacter clade can degrade dimethylsulfoniopropionate (DMSP) via competing pathways releasing either methanethiol (MeSH) or dimethyl sulfide (DMS). Deuterium-labeled [(2)H6]DMSP and the synthetic DMSP analogue dimethyltelluriopropionate (DMTeP) were used in feeding experiments with the Roseobacter clade members Phaeobacter gallaeciensis DSM 17395 and Ruegeria pomeroyi DSS-3, and their volatile metabolites were analyzed by closed-loop stripping and solid-phase microextraction coupled to GC-MS. Feeding experiments with [(2)H6]DMSP resulted in the incorporation of a deuterium label into MeSH and DMS. Knockout of relevant genes from the known DMSP demethylation pathway to MeSH showed in both species a residual production of [(2)H3]MeSH, suggesting that a second demethylation pathway is active. The role of DMSP degradation pathways for MeSH and DMS formation was further investigated by using the synthetic analogue DMTeP as a probe in feeding experiments with the wild-type strain and knockout mutants. Feeding of DMTeP to the R. pomeroyi knockout mutant resulted in a diminished, but not abolished production of demethylation pathway products. These results further corroborated the proposed second demethylation activity in R. pomeroyi. Isotopically labeled [(2)H3]methionine and (34)SO4 (2-), synthesized from elemental (34)S8, were tested to identify alternative sulfur sources besides DMSP for the MeSH production in P. gallaeciensis. Methionine proved to be a viable sulfur source for the MeSH volatiles, whereas incorporation of labeling from sulfate was not observed. Moreover, the utilization of selenite and selenate salts by marine alphaproteobacteria for the production of methylated selenium volatiles was explored and resulted in the production of numerous methaneselenol-derived volatiles via reduction and methylation. The pathway of selenate/selenite reduction, however, proved to be strictly separated from sulfate reduction.
Keywords: Roseobacter clade; dimethylsulfoniopropionate; selenium metabolism; sulfur metabolism; volatiles.
Figures




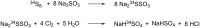


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
