3D-printed devices for continuous-flow organic chemistry
- PMID: 23766811
- PMCID: PMC3678713
- DOI: 10.3762/bjoc.9.109
3D-printed devices for continuous-flow organic chemistry
Abstract
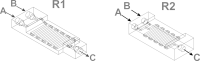
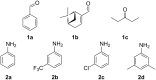
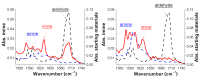
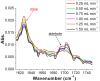
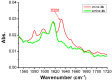
We present a study in which the versatility of 3D-printing is combined with the processing advantages of flow chemistry for the synthesis of organic compounds. Robust and inexpensive 3D-printed reactionware devices are easily connected using standard fittings resulting in complex, custom-made flow systems, including multiple reactors in a series with in-line, real-time analysis using an ATR-IR flow cell. As a proof of concept, we utilized two types of organic reactions, imine syntheses and imine reductions, to show how different reactor configurations and substrates give different products.
Keywords: 3D printing; flow IR; flow chemistry; imine reduction; imine synthesis; in-line analysis; millifluidics; reactionware.
Figures


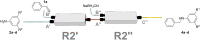
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
