How noise contributes to time-scale invariance of interval timing
- PMID: 23767576
- PMCID: PMC7015149
- DOI: 10.1103/PhysRevE.87.052717
How noise contributes to time-scale invariance of interval timing
Abstract
Time perception in the suprasecond range is crucial for fundamental cognitive processes such as decision making, rate calculation, and planning. In the vast majority of species, behavioral manipulations, and neurophysiological manipulations, interval timing is scale invariant: the time-estimation errors are proportional to the estimated duration. The origin and mechanisms of this fundamental property are unknown. We discuss the computational properties of a circuit consisting of a large number of (input) neural oscillators projecting on a small number of (output) coincidence detector neurons, which allows time to be coded by the pattern of coincidental activation of its inputs. We show that time-scale invariance emerges from the neural noise, such as small fluctuations in the firing patterns of its input neurons and in the errors with which information is encoded and retrieved by its output neurons. In this architecture, time-scale invariance is resistant to manipulations as it depends neither on the details of the input population nor on the distribution probability of noise.
Figures

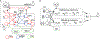
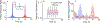
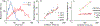

References
-
- Gallistel C, The organization of behavior (MIT Press, Cambridge, MA, 1990).
-
- Catania AC, “Reinforcement schedules and psychophysical judgments: A study of some temporal properties of behavior,” in The theory of reinforcement schedules, edited by Schoenfeld W (Appleton-Century-Crofts, New York, 1970) pp. 1–42.
-
- Roberts S, Journal of Experimental Psychology: Animal Behavior Processes 7, 242 (1981). - PubMed
-
- Buhusi C and Meck W, Nature Reviews Neuroscience 6, 755 (2005). - PubMed
-
- Mauk M and Buonomano D, Annu Rev Neurosci 27, 307 (2004). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
