Cloning, overexpression and biocatalytic exploration of a novel Baeyer-Villiger monooxygenase from Aspergillus fumigatus Af293
- PMID: 23767684
- PMCID: PMC3762062
- DOI: 10.1186/2191-0855-3-33
Cloning, overexpression and biocatalytic exploration of a novel Baeyer-Villiger monooxygenase from Aspergillus fumigatus Af293
Erratum in
-
Erratum: Cloning, overexpression and biocatalytic exploration of a novel Baeyer-Villiger monooxygenase from Aspergillus fumigatus Af293.AMB Express. 2014 Dec;4(1):62. doi: 10.1186/s13568-014-0062-7. Epub 2014 Sep 20. AMB Express. 2014. PMID: 26267109 Free PMC article. No abstract available.
Abstract
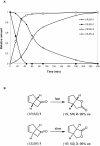
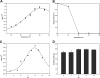
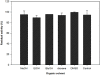
The presence of several putative Baeyer-Villiger Monooxygenases (BVMOs) encoding genes in Aspergillus fumigatus Af293 was demonstrated for the first time. One of the identified BVMO-encoding genes was cloned and successfully overexpressed fused to the cofactor regenerating enzyme phosphite dehydrogenase (PTDH). The enzyme named BVMOAf1 was extensively characterized in terms of its substrate scope and essential kinetic features. It showed high chemo-, regio- and stereoselectivity not only in the oxidation of asymmetric sulfides, (S)-sulfoxides were obtained with 99% ee, but also in the kinetic resolution of bicyclo[3.2.0]hept-2-en-6-one. This kinetic resolution process led to the production of (1S,5R) normal lactone and (1R,5S) abnormal lactone with a regioisomeric ratio of 1:1 and 99% ee each. Besides, different reaction conditions, such as pH, temperature and the presence of organic solvents, have been tested, revealing that BVMOAf1 is a relatively robust biocatalyst.
Figures

References
-
- Bastos Borges K, De Souza BW, Durán-Patrón R, Tallarico Pupo M, Sueli Bonato P, González Collado I. Stereoselective biotransformations using fungi as biocatalysts. Tet Asymm. 2009;3:385–397. doi: 10.1016/j.tetasy.2009.02.009. - DOI
-
- Brondani PB, De Gonzalo G, Fraaije MW, Andrade LH. Selective oxidations of organoboron compounds catalyzed by Baeyer-Villiger Monooxygenases. Adv Synth Catal. 2011;3:2169–2173. doi: 10.1002/adsc.201100029. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

