Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation
- PMID: 23767781
- PMCID: PMC3895110
- DOI: 10.1021/cr300463y
Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation
Conflict of interest statement
The authors declare no competing financial interest.
Figures

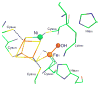
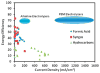

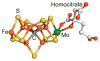

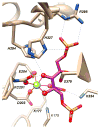
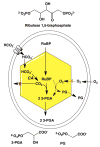
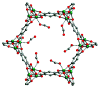
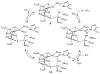

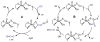
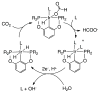

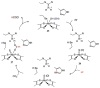
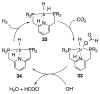
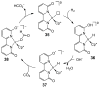
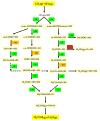
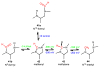

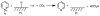

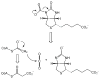
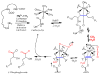
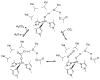

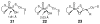
References
-
- Doney SC, Fabry VJ, Feely RA, Kleypas JA. Annu Rev Mar Sci. 2009;1:169. - PubMed
-
- Geider RJ, et al. Global Change Biol. 2001;7:849.
-
- Beer C, et al. Science. 2010;329:834. - PubMed
-
- Bell AT. Basic Research Needs, Catalysis for Energy. DOE Report. 2008 http://www.sc.doe.gov/bes/reports/files/CAT_rpt.pdf.
-
- Fontecilla-Camps JC, Volbeda A, Cavazza C, Nicolet Y. Chem Rev. 2007;107:4273. - PubMed
- Fontecilla-Camps JC, Amara P, Cavazza C, Nicolet Y, Volbeda A. Nature. 2009;460:814. - PubMed
- Lubitz W, Reijerse E, van Gastel M. Chem Rev. 2007;107:4331. - PubMed
- Barton BE, Rauchfuss TB. J Am Chem Soc. 2010;132:14877. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

