Interfacial bond-breaking electron transfer in mixed water-ethylene glycol solutions: reorganization energy and interplay between different solvent modes
- PMID: 23768162
- PMCID: PMC3725609
- DOI: 10.1021/jp405097c
Interfacial bond-breaking electron transfer in mixed water-ethylene glycol solutions: reorganization energy and interplay between different solvent modes
Abstract
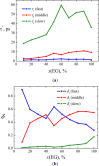
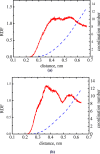
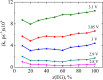
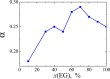
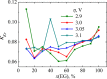
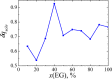
We explore solvent dynamics effects in interfacial bond breaking electron transfer in terms of a multimode approach and make an attempt to interpret challenging recent experimental results (the nonmonotonous behavior of the rate constant of electroreduction of S2O8(2-) from mixed water-EG solutions when increasing the EG fraction; see Zagrebin, P.A. et al. J. Phys. Chem. B 2010, 114, 311). The exact expansion of the solvent correlation function (calculated using experimental dielectric spectra) in a series predicts the splitting of solvent coordinate in three independent modes characterized by different relaxation times. This makes it possible to construct a 5D free-energy surface along three solvent coordinates and one intramolecular degree of freedom describing first electron transfer at the reduction of a peroxodisulphate anion. Classical molecular dynamics simulations were performed to study the solvation of a peroxodisulphate anion (S2O8(2-)) in oxidized and reduced states in pure water and ethylene glycol (EG) as well as mixed H2O-EG solutions. The solvent reorganization energy of the first electron-transfer step at the reduction of S2O8(2-) was calculated for several compositions of the mixed solution. This quantity was found to be significantly asymmetric. (The reorganization energies of reduction and oxidation differ from each other.) The averaged reorganization energy slightly increases with increasing the EG content in solution. This finding clearly indicates that for the reaction under study the static solvent effect no longer competes with solvent dynamics. Brownian dynamics simulations were performed to calculate the electron-transfer rate constants as a function of the solvent composition. The results of the simulations explain the experimental data, at least qualitatively.
Figures

References
-
- Nazmutdinov R. R.; Glukhov D. V.; Tsirlina G. A.; Petrii O. A. Molecular Description of the Persulfate Ion Reduction on a Mercury Electrode. Russ. J. Electrochem. 2002, 38, 720–731.
-
- Nazmutdinov R. R.; Glukhov D. V.; Petrii O. A.; Tsirlina G. A.; Botukhova G. N. Contemporary Understanding of the Peroxodisulfate Reduction at a Mercury Electrode. J. Electroanal. Chem. 2003, 552, 261–278.
-
- Kuznetsov A. M.Charge Transfer in Physics, Chemistry and Biology: The Physical Mechanism of Elementary Processes and Introduction to the theory; Gordon and Breach: Berkshire, U.K., 1995.
-
- Kuznetsov A. M.Stochastic and Dynamic Views of Chemical Reaction Kinetics in Solutions. Presses Polytechniques et Universitaires Romandes: Lausanne, 1999.
-
- Titova N. V.; Laurinavichute V. K.; Kuz’minova Z. V.; Tsirlina G. A. Electroreduction of Peroxodisulfate on Mercury in Mixed Water-Carbohydrate Media: The Interplay of Solvent Effects and Concentration-Dependent Structure of Reaction Layer. Chem. Phys. 2008, 352, 345–352.
LinkOut - more resources
Full Text Sources
Other Literature Sources

