Efficacy of vagus nerve stimulation as a treatment for medically intractable epilepsy in brain tumor patients. A case-controlled study using the VNS therapy Patient Outcome Registry
- PMID: 23768541
- PMCID: PMC3766475
- DOI: 10.1016/j.seizure.2013.04.017
Efficacy of vagus nerve stimulation as a treatment for medically intractable epilepsy in brain tumor patients. A case-controlled study using the VNS therapy Patient Outcome Registry
Abstract
Purpose: Vagus nerve stimulation (VNS) therapy is a procedure to control seizure frequency in patients with medically intractable epilepsy. However, there is no data on efficacy in the subset of these patients with brain tumors. The purpose of this study is to evaluate the efficacy of VNS therapy in patients with brain tumor-associated medically intractable epilepsy.
Methods: Data from the VNS therapy Patient Outcome Registry, maintained by the manufacturer of the device, Cyberonics Inc. (Houston, TX, USA), was queried to characterize the response of patients in whom a brain tumor was listed as the etiology of epilepsy. A case-control analysis was implemented and patient outcome was measured by Engel classification, median seizure response and responder rate (≥50% seizure reduction) using t-tests and chi-squared tests.
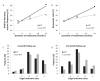
Results: In 107 patients with an epilepsy etiology related to a brain tumor, seizure reduction was 45% at 3 months and 79% at 24 months with a responder rate of 48% at 3 months and 79% at 24 months. There was no statistical difference in seizure reduction compared with 326 case-control patients from the registry without brain tumors. There was no significant difference in anti-epileptic drug (AED) usage from baseline to 24 months post implant in either group.
Conclusions: VNS therapy is equally effective in patients who suffer seizures secondary to brain tumors as in patients without history of a brain tumor. VNS therapy is a viable treatment option for patients with brain tumor associated medically intractable epilepsy, assuming cytoreductive and other adjuvant therapies have been fully explored.
Keywords: Brain tumors; Medically intractable epilepsy; Seizures; Vagus nerve stimulation.
Copyright © 2013 British Epilepsy Association. Published by Elsevier Ltd. All rights reserved.
Figures


References
-
- Berg AT, Shinnar S, Levy SR, Testa FM, Smith-Rapaport S, Beckerman B, et al. Two-year remission and subsequent relapse in children with newly diagnosed epilepsy. Epilepsia. 2001;42:1553–62. - PubMed
-
- Berg AT, Langfitt J, Shinnar S, Vickrey BG, Sperling MR, Walczak T, et al. How long does it take for partial epilepsy to become intractable? Neurology. 2003;60:186–90. - PubMed
-
- Benbadis SR, Tatum WO, Vale FL. When drugs don’t work: an algorithmic approach to medically intractable epilepsy. Neurology. 2000;55:1780–4. - PubMed
-
- Baaj AA, Benbadis SR, Tatum WO, Vale FL. Trends in the use of vagus nerve stimulation for epilepsy: analysis of a nationwide database. Neurosurgical Focus. 2008;25:E10. - PubMed
-
- Ben-Menachem E, Manon-Espaillat R, Ristanovic R, Wilder BJ, Stefan H, Mirza W, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35:616–26. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

