Imaging challenges in biomaterials and tissue engineering
- PMID: 23768903
- PMCID: PMC3799904
- DOI: 10.1016/j.biomaterials.2013.05.033
Imaging challenges in biomaterials and tissue engineering
Abstract
Biomaterials are employed in the fields of tissue engineering and regenerative medicine (TERM) in order to enhance the regeneration or replacement of tissue function and/or structure. The unique environments resulting from the presence of biomaterials, cells, and tissues result in distinct challenges in regards to monitoring and assessing the results of these interventions. Imaging technologies for three-dimensional (3D) analysis have been identified as a strategic priority in TERM research. Traditionally, histological and immunohistochemical techniques have been used to evaluate engineered tissues. However, these methods do not allow for an accurate volume assessment, are invasive, and do not provide information on functional status. Imaging techniques are needed that enable non-destructive, longitudinal, quantitative, and three-dimensional analysis of TERM strategies. This review focuses on evaluating the application of available imaging modalities for assessment of biomaterials and tissue in TERM applications. Included is a discussion of limitations of these techniques and identification of areas for further development.
Published by Elsevier Ltd.
Figures



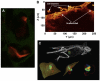

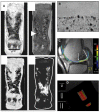
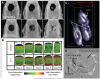
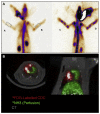
References
-
- Multi-agency tissue engineering science: a foundation for the future. Advancing tissue science and engineering: a multi-agency strategic plan. Jun, 2007. - PubMed
-
- Assmann A, Akhyari P, Delfs C, Flogel U, Jacoby C, Kamiya H, et al. Development of a growing rat model for the in vivo assessment of engineered aortic conduits. J Surg Res. 2012;176:367–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

