Comparative effectiveness of surfactant preparations in premature infants
- PMID: 23769501
- PMCID: PMC3779477
- DOI: 10.1016/j.jpeds.2013.04.053
Comparative effectiveness of surfactant preparations in premature infants
Abstract
Objective: To compare effectiveness of 3 surfactant preparations (beractant, calfactant, and poractant alfa) in premature infants for preventing 3 outcomes: (1) air leak syndromes; (2) death; and (3) bronchopulmonary dysplasia (BPD) or death (composite outcome).
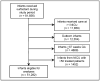
Study design: We conducted a comparative effectiveness study of premature infants admitted to 322 neonatal intensive care units in the US from 2005-2010 who were treated with beractant, calfactant, or poractant alfa. We compared the incidence of air leak syndromes, death, and BPD or death, adjusting for gestational age (GA), antenatal steroids, discharge year, and small for GA status.
Results: A total of 51282 infants received surfactant; 40% received beractant, 30% calfactant, and 30% poractant alfa. Median birth weight was 1435 g (IQR 966-2065); median GA was 30 weeks (27-33). On adjusted analysis, we observed a similar risk of air leak syndromes (calfactant vs beractant OR = 1.17 [95% CI: 0.95, 1.43]; calfactant vs poractant OR = 1.23 [0.98, 1.56]; beractant vs poractant OR = 1.06 [0.87, 1.29]), death (calfactant vs beractant OR = 1.14 [0.93, 1.39]; calfactant vs poractant OR = 0.98 [0.78, 1.23]; beractant vs poractant OR = 0.86 [0.72, 1.04]), and BPD or death (calfactant vs beractant OR = 1.08 [0.93, 1.26]; calfactant vs poractant OR = 1.19 [1.00, 1.41]; beractant vs poractant OR = 1.10 [0.96, 1.27]).
Conclusions: Beractant, calfactant, and poractant alfa demonstrated similar effectiveness in prevention of air leak syndromes, death, and BPD or death in premature infants when adjusted for site. Previously described differences in mortality between surfactants likely do not represent true differences in effectiveness but may relate to site variation in outcomes.
Keywords: BPD; Bronchopulmonary dysplasia; GA; Gestational age; NICU; Neonatal intensive care unit; RDS; Respiratory distress syndrome.
Copyright © 2013 Mosby, Inc. All rights reserved.
Conflict of interest statement
The other authors declare no conflicts of interest.
Figures
References
-
- Engle WA. Surfactant-replacement therapy for respiratory distress in the preterm and term neonate. Pediatrics. 2008;121:419–32. - PubMed
-
- Trembath AN, Clark RH, Bloom BT, Smith PB, Bose C, Laughon M. Trends in surfactant use in the United States: changes in clinical practice. E J Neonatology Res. 2011;1:23–30.
-
- Ramanathan R, Rasmussen MR, Gerstmann DR, Finer N, Sekar K. A randomized, multicenter masked comparison trial of poractant alfa (Curosurf) versus beractant (Survanta) in the treatment of respiratory distress syndrome in preterm infants. J Perinatol. 2004;21:109–19. - PubMed
-
- Bloom BT, Clark RH. Comparison of Infasurf (calfactant) and Survanta (beractant) in the prevention and treatment of respiratory distress syndrome. Pediatrics. 2005;116:392–9. - PubMed
-
- Bloom BT, Kattwinkel J, Hall RT, Delmore PM, Egan EA, Trout JR, et al. Comparison of Infasurf (calf lung surfactant extract) to Survanta (Beractant) in the treatment and prevention of respiratory distress syndrome. Pediatrics. 1997;100:31–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources