Cells of renin lineage are progenitors of podocytes and parietal epithelial cells in experimental glomerular disease
- PMID: 23769837
- PMCID: PMC3730767
- DOI: 10.1016/j.ajpath.2013.04.024
Cells of renin lineage are progenitors of podocytes and parietal epithelial cells in experimental glomerular disease
Abstract
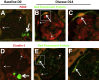
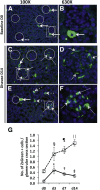
Glomerular injury leads to podocyte loss, a process directly underlying progressive glomerular scarring and decline of kidney function. The inherent repair process is limited by the inability of podocytes to regenerate. Cells of renin lineage residing alongside glomerular capillaries are reported to have progenitor capacity. We investigated whether cells of renin lineage can repopulate the glomerulus after podocyte injury and serve as glomerular epithelial cell progenitors. Kidney cells expressing renin were genetically fate-mapped in adult Ren1cCreER×Rs-tdTomato-R, Ren1cCre×Rs-ZsGreen-R, and Ren1dCre×Z/EG reporter mice. Podocyte depletion was induced in all three cell-specific reporter mice by cytotoxic anti-podocyte antibodies. After a decrease in podocyte number, a significant increase in the number of labeled cells of renin lineage was observed in glomeruli in a focal distribution along Bowman's capsule, within the glomerular tuft, or in both locations. A subset of cells lining Bowman's capsule activated expression of the glomerular parietal epithelial cell markers paired box protein PAX2 and claudin-1. A subset of labeled cells within the glomerular tuft expressed the podocyte markers Wilms tumor protein 1, nephrin, podocin, and synaptopodin. Neither renin mRNA nor renin protein was detected de novo in diseased glomeruli. These findings provide initial evidence that cells of renin lineage may enhance glomerular regeneration by serving as progenitors for glomerular epithelial cells in glomerular disease characterized by podocyte depletion.
Copyright © 2013 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





Comment in
-
Podocyte regeneration: who can become a podocyte?Am J Pathol. 2013 Aug;183(2):333-5. doi: 10.1016/j.ajpath.2013.04.009. Epub 2013 May 29. Am J Pathol. 2013. PMID: 23727347
References
-
- Foley R.N., Collins A.J. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol. 2007;18:2644–2648. - PubMed
-
- Nakamura T., Ushiyama C., Suzuki S., Hara M., Shimada N., Ebihara I., Koide H. Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant. 2000;15:1379–1383. - PubMed
-
- Petermann A.T., Pippin J., Krofft R., Blonski M., Griffin S., Durvasula R., Shankland S.J. Viable podocytes detach in experimental diabetic nephropathy: potential mechanism underlying glomerulosclerosis. Nephron Exp Nephrol. 2004;98:e114–e123. - PubMed
-
- Lerco M.M., Macedo C.S., Silva R.J., Pinheiro Dde O., Spadella C.T. The number of podocyte and slit diaphragm is decreased in experimental diabetic nephropathy. Acta Cir Bras. 2006;21:87–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RC1 DK84077/DK/NIDDK NIH HHS/United States
- R24 DK94768/DK/NIDDK NIH HHS/United States
- P30 CA016056/CA/NCI NIH HHS/United States
- R01 DK056799/DK/NIDDK NIH HHS/United States
- R21 CA121212/CA/NCI NIH HHS/United States
- R01-DK056799/DK/NIDDK NIH HHS/United States
- RC1 DK087389/DK/NIDDK NIH HHS/United States
- R21-DK081835/DK/NIDDK NIH HHS/United States
- R24 DK094768/DK/NIDDK NIH HHS/United States
- R21 DK081835/DK/NIDDK NIH HHS/United States
- R01 DK084077/DK/NIDDK NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- R01-HL048459/HL/NHLBI NIH HHS/United States
- R21-CA121212/CA/NCI NIH HHS/United States
- R01 DK87389/DK/NIDDK NIH HHS/United States
- P30-CA016056/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

