Study in Parkinson disease of exercise (SPARX): translating high-intensity exercise from animals to humans
- PMID: 23770108
- PMCID: PMC3769494
- DOI: 10.1016/j.cct.2013.06.002
Study in Parkinson disease of exercise (SPARX): translating high-intensity exercise from animals to humans
Abstract
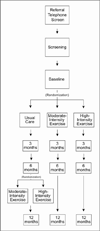
A burgeoning literature suggests that exercise has a therapeutic benefit in persons with Parkinson disease (PD) and in animal models of PD, especially when animals exercise at high intensity. If exercise is to be prescribed as "first-line" or "add-on" therapy in patients with PD, we must demonstrate its efficacy and dose-response effects through testing phases similar to those used in the testing of pharmacologic agents. The SPARX Trial is a multicenter, randomized, controlled, single-blinded, Phase II study that we designed to test the feasibility of using high-intensity exercise to modify symptoms of PD and to simultaneously test the nonfutility of achieving a prespecified change in patients' motor scores on the Unified Parkinson Disease Rating Scale (UPDRS). The trial began in May 2102 and is in the process of screening, enrolling, and randomly assigning 126 patients with early-stage PD to 1 of 3 groups: usual care (wait-listed controls), moderate-intensity exercise (4 days/week at 60%-65% maximal heart rate [HRmax]), or high-intensity exercise (4 days/week at 80%-85% HRmax). At 6-month follow-up, the trial is randomly reassigning usual care participants to a moderate-intensity or high-intensity exercise group for the remaining 6 months. The goals of the Phase II trial are to determine if participants can exercise at moderate and high intensities; to determine if either exercise yields benefits consistent with meaningful clinical change (nonfutility); and to document safety and attrition. The advantage of using a non-futility approach allows us to efficiently determine if moderate- or high-intensity exercise warrants further large-scale investigation in PD.
Trial registration: ClinicalTrials.gov NCT01506479.
Keywords: Exercise; Futility; Parkinson disease; Phase II; Randomized controlled trial; Unified Parkinson Disease Rating Scale.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
References
-
- Miyai I, et al. Treadmill training with body weight support: its effect on Parkinson's disease. Arch Phys Med Rehabil. 2000;81(7):849–852. - PubMed
-
- Schenkman M, et al. Endurance exercise training to improve economy of movement of people with Parkinson disease: three case reports. Phys Ther. 2008;88(1):63–76. - PubMed
-
- Ridgel AL, Vitek JL, Alberts JL. Forced, not voluntary, exercise improves motor function in Parkinson's disease patients. Neurorehabil Neural Repair. 2009;23(6):600–608. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

