Porcine hemagglutinating encephalomyelitis virus induces apoptosis in a porcine kidney cell line via caspase-dependent pathways
- PMID: 23770152
- PMCID: PMC7114423
- DOI: 10.1016/j.virusres.2013.05.019
Porcine hemagglutinating encephalomyelitis virus induces apoptosis in a porcine kidney cell line via caspase-dependent pathways
Abstract
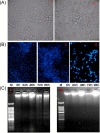
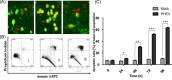
Porcine hemagglutinating encephalomyelitis is an acute, highly contagious disease in piglets that is caused by the porcine hemagglutinating encephalomyelitis virus (PHEV). However, the pathogenesis of PHEV and the relationship between PHEV and the host cells are not fully understood. In this study, we investigated whether the PHEV-induced cytopathic effect (CPE) was caused by apoptosis. Replication of PHEV in a porcine kidney-derived cell line (PK-15 cells) caused an extensive CPE, leading to the destruction of the entire monolayer and the death of the infected cells. Staining with Hoechst 33,342 revealed morphological changes in the nuclei and chromatin fragmentation. In addition, PHEV caused DNA fragmentation detectable by agarose gel electrophoresis 48h post-infection, increasing with the incubation time. The percentage of apoptotic cells increased with the incubation time and reached a maximum at 96h post-infection, as determined using flow cytometry and fluorescence microscopy of cells that were stained with annexin V-FITC and propidium iodide (PI). Moreover, as is commonly observed for coronavirus infections of other animals, the activities of the effecter caspase, caspase-3, and the initiator caspases, caspase-8 and caspase-9, which are representative factors in the death receptor-mediated apoptotic pathway and the mitochondrial apoptotic pathway, respectively, were increased in PHEV-infected PK-15 cells. Moreover, the tripeptide pan-ICE (caspase) inhibitor Z-VAD-FMK blocked PHEV-induced apoptosis but did not have an effect on virus production by 96h post-infection. These results suggested that PHEV induces apoptosis in PK-15 cells via a caspase-dependent pathway. Apoptotic death of infected cells is detrimental to animals because it causes cell and tissue destruction. Although the pathological characteristics of PHEV are largely unknown, apoptosis may be the pathological basis of the lesions resulting from PHEV infection.
Keywords: Apoptosis; Caspase; PK-15 cells; Procine haemagglutinating encephalomyelitis virus.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

