Glutathione redox dynamics and expression of glutathione-related genes in the developing embryo
- PMID: 23770340
- PMCID: PMC3823629
- DOI: 10.1016/j.freeradbiomed.2013.06.011
Glutathione redox dynamics and expression of glutathione-related genes in the developing embryo
Abstract
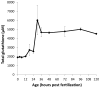
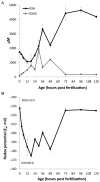
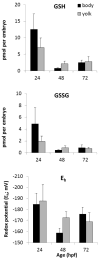
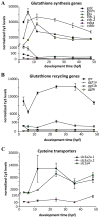
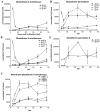
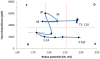
Embryonic development involves dramatic changes in cell proliferation and differentiation that must be highly coordinated and tightly regulated. Cellular redox balance is critical for cell fate decisions, but it is susceptible to disruption by endogenous and exogenous sources of oxidative stress. The most abundant endogenous nonprotein antioxidant defense molecule is the tripeptide glutathione (γ-glutamylcysteinylglycine, GSH), but the ontogeny of GSH concentration and redox state during early life stages is poorly understood. Here, we describe the GSH redox dynamics during embryonic and early larval development (0-5 days postfertilization) in the zebrafish (Danio rerio), a model vertebrate embryo. We measured reduced and oxidized glutathione using HPLC and calculated the whole embryo total glutathione (GSHT) concentrations and redox potentials (Eh) over 0-120 h of zebrafish development (including mature oocytes, fertilization, midblastula transition, gastrulation, somitogenesis, pharyngula, prehatch embryos, and hatched eleutheroembryos). GSHT concentration doubled between 12h postfertilization (hpf) and hatching. The GSH Eh increased, becoming more oxidizing during the first 12h, and then oscillated around -190 mV through organogenesis, followed by a rapid change, associated with hatching, to a more negative (more reducing) Eh (-220 mV). After hatching, Eh stabilized and remained steady through 120 hpf. The dynamic changes in GSH redox status and concentration defined discrete windows of development: primary organogenesis, organ differentiation, and larval growth. We identified the set of zebrafish genes involved in the synthesis, utilization, and recycling of GSH, including several novel paralogs, and measured how expression of these genes changes during development. Ontogenic changes in the expression of GSH-related genes support the hypothesis that GSH redox state is tightly regulated early in development. This study provides a foundation for understanding the redox regulation of developmental signaling and investigating the effects of oxidative stress during embryogenesis.
Keywords: Antioxidant; Embryonic development; Free radicals; GSH; GSSG; Gcl; Gclc; Gclm; Gene expression; Glutathione; Gss; Oxidative stress; PCA; ROS; Redox; Zebrafish; glutamate–cysteine ligase; glutamate–cysteine ligase catalytic subunit; glutamate–cysteine ligase modifier subunit; glutathione disulfide; glutathione synthetase; perchloric acid; reactive oxygen species; reduced glutathione.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

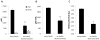
Similar articles
-
Pancreatic beta cells are a sensitive target of embryonic exposure to butylparaben in zebrafish (Danio rerio).Birth Defects Res. 2018 Jul 3;110(11):933-948. doi: 10.1002/bdr2.1215. Epub 2018 Mar 8. Birth Defects Res. 2018. PMID: 29516647 Free PMC article.
-
Inhibition of glutathione biosynthesis alters compartmental redox status and the thiol proteome in organogenesis-stage rat conceptuses.Free Radic Biol Med. 2013 Oct;63:325-37. doi: 10.1016/j.freeradbiomed.2013.05.040. Epub 2013 Jun 2. Free Radic Biol Med. 2013. PMID: 23736079 Free PMC article.
-
Systemic and strict regulation of the glutathione redox state in mitochondria and cytosol is needed for zebrafish ontogeny.Biochim Biophys Acta Gen Subj. 2024 Jun;1868(6):130603. doi: 10.1016/j.bbagen.2024.130603. Epub 2024 Mar 21. Biochim Biophys Acta Gen Subj. 2024. PMID: 38521470
-
Glutathione synthesis.Biochim Biophys Acta. 2013 May;1830(5):3143-53. doi: 10.1016/j.bbagen.2012.09.008. Epub 2012 Sep 17. Biochim Biophys Acta. 2013. PMID: 22995213 Free PMC article. Review.
-
Glutathione--linking cell proliferation to oxidative stress.Free Radic Biol Med. 2015 Dec;89:1154-64. doi: 10.1016/j.freeradbiomed.2015.09.023. Epub 2015 Nov 3. Free Radic Biol Med. 2015. PMID: 26546102 Review.
Cited by
-
Ribose-5-phosphate isomerase A overexpression promotes liver cancer development in transgenic zebrafish via activation of ERK and β-catenin pathways.Carcinogenesis. 2019 May 14;40(3):461-473. doi: 10.1093/carcin/bgy155. Carcinogenesis. 2019. PMID: 30418535 Free PMC article.
-
Maternal transfer of nanoplastics to offspring in zebrafish (Danio rerio): A case study with nanopolystyrene.Sci Total Environ. 2018 Dec 1;643:324-334. doi: 10.1016/j.scitotenv.2018.06.186. Epub 2018 Jun 22. Sci Total Environ. 2018. PMID: 29940444 Free PMC article.
-
Pancreatic beta cells are a sensitive target of embryonic exposure to butylparaben in zebrafish (Danio rerio).Birth Defects Res. 2018 Jul 3;110(11):933-948. doi: 10.1002/bdr2.1215. Epub 2018 Mar 8. Birth Defects Res. 2018. PMID: 29516647 Free PMC article.
-
Nrf2a modulates the embryonic antioxidant response to perfluorooctanesulfonic acid (PFOS) in the zebrafish, Danio rerio.Aquat Toxicol. 2018 May;198:92-102. doi: 10.1016/j.aquatox.2018.02.010. Epub 2018 Feb 20. Aquat Toxicol. 2018. PMID: 29524743 Free PMC article.
-
Programmed Effects in Neurobehavior and Antioxidative Physiology in Zebrafish Embryonically Exposed to Cadmium: Observations and Hypothesized Adverse Outcome Pathway Framework.Int J Mol Sci. 2016 Nov 2;17(11):1830. doi: 10.3390/ijms17111830. Int J Mol Sci. 2016. PMID: 27827847 Free PMC article.
References
-
- Hernandez-Garcia D, Wood CD, Castro-Obregon S, Covarrubias L. Reactive oxygen species: A radical role in development? Free radical biology & medicine. 2010;49:130–143. - PubMed
-
- Covarrubias L, Hernandez-Garcia D, Schnabel D, Salas-Vidal E, Castro-Obregon S. Function of reactive oxygen species during animal development: passive or active? Developmental biology. 2008;320:1–11. - PubMed
-
- Coffman JA, Davidson EH. Oral-aboral axis specification in the sea urchin embryo. I. Axis entrainment by respiratory asymmetry. Developmental biology. 2001;230:18–28. - PubMed
-
- Coffman JA, Denegre JM. Mitochondria, redox signaling and axis specification in metazoan embryos. Developmental biology. 2007;308:266–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

