Activity-dependent phosphorylation of MeCP2 threonine 308 regulates interaction with NCoR
- PMID: 23770587
- PMCID: PMC3922283
- DOI: 10.1038/nature12348
Activity-dependent phosphorylation of MeCP2 threonine 308 regulates interaction with NCoR
Abstract
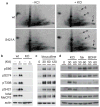
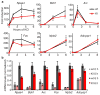
Rett syndrome (RTT) is an X-linked human neurodevelopmental disorder with features of autism and severe neurological dysfunction in females. RTT is caused by mutations in methyl-CpG-binding protein 2 (MeCP2), a nuclear protein that, in neurons, regulates transcription, is expressed at high levels similar to that of histones, and binds to methylated cytosines broadly across the genome. By phosphotryptic mapping, we identify three sites (S86, S274 and T308) of activity-dependent MeCP2 phosphorylation. Phosphorylation of these sites is differentially induced by neuronal activity, brain-derived neurotrophic factor, or agents that elevate the intracellular level of 3',5'-cyclic AMP (cAMP), indicating that MeCP2 may function as an epigenetic regulator of gene expression that integrates diverse signals from the environment. Here we show that the phosphorylation of T308 blocks the interaction of the repressor domain of MeCP2 with the nuclear receptor co-repressor (NCoR) complex and suppresses the ability of MeCP2 to repress transcription. In knock-in mice bearing the common human RTT missense mutation R306C, neuronal activity fails to induce MeCP2 T308 phosphorylation, suggesting that the loss of T308 phosphorylation might contribute to RTT. Consistent with this possibility, the mutation of MeCP2 T308A in mice leads to a decrease in the induction of a subset of activity-regulated genes and to RTT-like symptoms. These findings indicate that the activity-dependent phosphorylation of MeCP2 at T308 regulates the interaction of MeCP2 with the NCoR complex, and that RTT in humans may be due, in part, to the loss of activity-dependent MeCP2 T308 phosphorylation and a disruption of the phosphorylation-regulated interaction of MeCP2 with the NCoR complex.
Conflict of interest statement
Figures
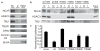

References
-
- Nan X, Campoy FJ, Bird A. Mecp2 Is a Transcriptional Repressor with Abundant Binding Sites in Genomic Chromatin. Cell. 1997;88:471–481. - PubMed
-
- Nan X, et al. Transcriptional Repression by the Methyl-Cpg-Binding Protein Mecp2 Involves a Histone Deacetylase Complex. Nature. 1998;393:386–389. - PubMed
-
- Amir RE, et al. Rett Syndrome Is Caused by Mutations in X-Linked Mecp2, Encoding Methyl-Cpg-Binding Protein 2. Nat Genet. 1999;23:185–188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

