Assessment of homology templates and an anesthetic binding site within the γ-aminobutyric acid receptor
- PMID: 23770602
- PMCID: PMC3823821
- DOI: 10.1097/ALN.0b013e31829e47e3
Assessment of homology templates and an anesthetic binding site within the γ-aminobutyric acid receptor
Abstract
Background: Anesthetics mediate portions of their activity via modulation of the γ-aminobutyric acid receptor (GABAaR). Although its molecular structure remains unknown, significant progress has been made toward understanding its interactions with anesthetics via molecular modeling.
Methods: The structure of the torpedo acetylcholine receptor (nAChRα), the structures of the α4 and β2 subunits of the human nAChR, the structures of the eukaryotic glutamate-gated chloride channel (GluCl), and the prokaryotic pH-sensing channels, from Gloeobacter violaceus and Erwinia chrysanthemi, were aligned with the SAlign and 3DMA algorithms. A multiple sequence alignment from these structures and those of the GABAaR was performed with ClustalW. The Modeler and Rosetta algorithms independently created three-dimensional constructs of the GABAaR from the GluCl template. The CDocker algorithm docked a congeneric series of propofol derivatives into the binding pocket and scored calculated binding affinities for correlation with known GABAaR potentiation EC50s.
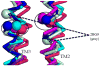
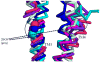
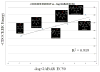
Results: Multiple structure alignments of templates revealed a clear consensus of residue locations relevant to anesthetic effects except for torpedo nAChR. Within the GABAaR models generated from GluCl, the residues notable for modulating anesthetic action within transmembrane segments 1, 2, and 3 converged on the intersubunit interface between α and β subunits. Docking scores of a propofol derivative series into this binding site showed strong linear correlation with GABAaR potentiation EC50.
Conclusion: Consensus structural alignment based on homologous templates revealed an intersubunit anesthetic binding cavity within the transmembrane domain of the GABAaR, which showed a correlation of ligand docking scores with experimentally measured GABAaR potentiation.
Similar articles
-
Insights Into Receptor-Based Anesthetic Pharmacophores and Anesthetic-Protein Interactions.Methods Enzymol. 2018;602:77-95. doi: 10.1016/bs.mie.2018.01.004. Epub 2018 Mar 2. Methods Enzymol. 2018. PMID: 29588042
-
Common Anesthetic-binding Site for Inhibition of Pentameric Ligand-gated Ion Channels.Anesthesiology. 2016 Mar;124(3):664-73. doi: 10.1097/ALN.0000000000001005. Anesthesiology. 2016. PMID: 26756520 Free PMC article.
-
Enantiomeric barbiturates bind distinct inter- and intrasubunit binding sites in a nicotinic acetylcholine receptor (nAChR).J Biol Chem. 2017 Oct 20;292(42):17258-17271. doi: 10.1074/jbc.M117.808592. Epub 2017 Sep 6. J Biol Chem. 2017. PMID: 28878016 Free PMC article.
-
Structural models of ligand-gated ion channels: sites of action for anesthetics and ethanol.Alcohol Clin Exp Res. 2014 Mar;38(3):595-603. doi: 10.1111/acer.12283. Epub 2013 Oct 24. Alcohol Clin Exp Res. 2014. PMID: 24164436 Free PMC article. Review.
-
Assembly of nicotinic and other Cys-loop receptors.J Neurochem. 2011 Mar;116(5):734-41. doi: 10.1111/j.1471-4159.2010.07060.x. Epub 2011 Jan 13. J Neurochem. 2011. PMID: 21214570 Review.
Cited by
-
GABA(A) receptor transmembrane amino acids are critical for alcohol action: disulfide cross-linking and alkyl methanethiosulfonate labeling reveal relative location of binding sites.J Neurochem. 2014 Feb;128(3):363-75. doi: 10.1111/jnc.12476. Epub 2013 Oct 28. J Neurochem. 2014. PMID: 24117469 Free PMC article.
-
Pentameric Ligand-gated Ion Channels : Insights from Computation.Mol Simul. 2014 Apr 17;40(10-11):821-829. doi: 10.1080/08927022.2014.896462. Mol Simul. 2014. PMID: 25931676 Free PMC article.
-
An In Vitro Model for Identifying Cardiac Side Effects of Anesthetics.Anesth Analg. 2020 Jan;130(1):e1-e4. doi: 10.1213/ANE.0000000000003757. Anesth Analg. 2020. PMID: 30198930 Free PMC article.
-
Modulation of α1β3γ2 GABAA receptors expressed in X. laevis oocytes using a propofol photoswitch tethered to the transmembrane helix.Proc Natl Acad Sci U S A. 2021 Feb 23;118(8):e2008178118. doi: 10.1073/pnas.2008178118. Proc Natl Acad Sci U S A. 2021. PMID: 33593898 Free PMC article.
-
A Novel Bifunctional Alkylphenol Anesthetic Allows Characterization of γ-Aminobutyric Acid, Type A (GABAA), Receptor Subunit Binding Selectivity in Synaptosomes.J Biol Chem. 2016 Sep 23;291(39):20473-86. doi: 10.1074/jbc.M116.736975. Epub 2016 Jul 26. J Biol Chem. 2016. PMID: 27462076 Free PMC article.
References
-
- Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor β3 subunit. Faseb J. 2003;17:250–2. - PubMed
-
- Bertaccini E, Trudell JR. Predicting the transmembrane secondary structure of ligand-gated ion channels. Protein Eng. 2002;15:443–54. - PubMed
-
- Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;424:949–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources