The nuclear receptor LXRα controls the functional specialization of splenic macrophages
- PMID: 23770640
- PMCID: PMC3720686
- DOI: 10.1038/ni.2622
The nuclear receptor LXRα controls the functional specialization of splenic macrophages
Abstract
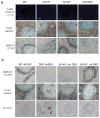
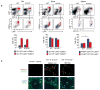
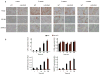
Macrophages are professional phagocytic cells that orchestrate innate immune responses and have considerable phenotypic diversity at different anatomical locations. However, the mechanisms that control the heterogeneity of tissue macrophages are not well characterized. Here we found that the nuclear receptor LXRα was essential for the differentiation of macrophages in the marginal zone (MZ) of the spleen. LXR-deficient mice were defective in the generation of MZ and metallophilic macrophages, which resulted in abnormal responses to blood-borne antigens. Myeloid-specific expression of LXRα or adoptive transfer of wild-type monocytes restored the MZ microenvironment in LXRα-deficient mice. Our results demonstrate that signaling via LXRα in myeloid cells is crucial for the generation of splenic MZ macrophages and identify an unprecedented role for a nuclear receptor in the generation of specialized macrophage subsets.
Conflict of interest statement
The authors declare no competing financial interests
Figures

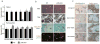



References
-
- Taylor PR, et al. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. - PubMed
-
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. - PubMed
-
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

