Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma
- PMID: 23770775
- PMCID: PMC3743463
- DOI: 10.1182/blood-2013-03-490565
Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma
Abstract
An obstacle to cancer immunotherapy has been that the affinity of T-cell receptors (TCRs) for antigens expressed in tumors is generally low. We initiated clinical testing of engineered T cells expressing an affinity-enhanced TCR against HLA-A*01-restricted MAGE-A3. Open-label protocols to test the TCRs for patients with myeloma and melanoma were initiated. The first two treated patients developed cardiogenic shock and died within a few days of T-cell infusion, events not predicted by preclinical studies of the high-affinity TCRs. Gross findings at autopsy revealed severe myocardial damage, and histopathological analysis revealed T-cell infiltration. No MAGE-A3 expression was detected in heart autopsy tissues. Robust proliferation of the engineered T cells in vivo was documented in both patients. A beating cardiomyocyte culture generated from induced pluripotent stem cells triggered T-cell killing, which was due to recognition of an unrelated peptide derived from the striated muscle-specific protein titin. These patients demonstrate that TCR-engineered T cells can have serious and not readily predictable off-target and organ-specific toxicities and highlight the need for improved methods to define the specificity of engineered TCRs.
Figures


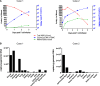
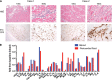

Comment in
-
Genetic engineering of T-cell receptors: TCR takes to titin.Blood. 2013 Aug 8;122(6):853-4. doi: 10.1182/blood-2013-06-509604. Blood. 2013. PMID: 23929832 Free PMC article.
References
-
- Schumacher TN. T-cell-receptor gene therapy. Nat Rev Immunol. 2002;2(7):512–519. - PubMed
-
- Purbhoo MA, Sutton DH, Brewer JE, et al. Quantifying and imaging NY-ESO-1/LAGE-1-derived epitopes on tumor cells using high affinity T cell receptors. J Immunol. 2006;176(12):7308–7316. - PubMed
-
- Cameron BJ, Gerry AB, Dukes J, et al. Identification of titin as an alternative specificity for engineered T cells expressing an enhanced affinity MAGE A3 TCR. Sci Transl Med. In press.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

