Heterogeneity and immunophenotypic plasticity of malignant cells in human liposarcomas
- PMID: 23770802
- PMCID: PMC3737386
- DOI: 10.1016/j.scr.2013.04.011
Heterogeneity and immunophenotypic plasticity of malignant cells in human liposarcomas
Abstract
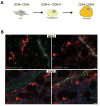
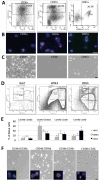
Liposarcomas are tumors arising in white adipose tissue (WAT) with avidity for local recurrence. Aggressive dedifferentiated liposarcomas (DDLS) may arise from well-differentiated subtypes (WDLS) upon disease progression, however, this key issue is unresolved due in large part to knowledge gaps about liposarcoma cellular composition. Here, we wished to improve insights into liposarcoma cellular hierarchy. Tumor section analysis indicated that the populations, distinguishable based on the expression of CD34 (a marker of adipocyte progenitors) and CD36 (a marker of adipocyte differentiation), occupy distinct intra-tumoral locations in both WDLS and DDLS. Taking advantage of these markers, we separated cells from a panel of fresh human surgical specimens by fluorescence-activated cell sorting (FACS). Based on chromosome analysis and the culture phenotypes of the composing populations, we demonstrate that malignant cells comprise four mesenchymal populations distinguished by the expression of CD34 and CD36, while vascular (CD31+) and hematopoietic (CD45+) components are non-neoplastic. Finally, we show that mouse xenografts are derivable from both CD36-negative and CD36-positive DDLS cells, and that each population recreates the heterogeneity of CD36 expression in vivo. Combined, our results show that malignant cells in WDLS and DDLS can be classified according to distinct stages of adipogenesis and indicate immunophenotypic plasticity of malignant liposarcoma cells.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Immunoreactivity of p53, mdm2, and p21WAF1 in dedifferentiated liposarcoma: special emphasis on the distinct immunophenotype of the well-differentiated component.Int J Surg Pathol. 2001 Apr;9(2):99-109. doi: 10.1177/106689690100900203. Int J Surg Pathol. 2001. PMID: 11484509
-
Evaluation of well-differentiated/de-differentiated liposarcomas by high-resolution oligonucleotide array-based comparative genomic hybridization.Genes Chromosomes Cancer. 2011 Feb;50(2):95-112. doi: 10.1002/gcc.20835. Genes Chromosomes Cancer. 2011. PMID: 21117066
-
Retroperitoneal liposarcomas: follow-up analysis of dedifferentiation after clinicopathologic reexamination of 86 liposarcomas and malignant fibrous histiocytomas.Cancer. 2006 Jun 15;106(12):2725-33. doi: 10.1002/cncr.21933. Cancer. 2006. PMID: 16688768
-
Clinical and Molecular Spectrum of Liposarcoma.J Clin Oncol. 2018 Jan 10;36(2):151-159. doi: 10.1200/JCO.2017.74.9598. Epub 2017 Dec 8. J Clin Oncol. 2018. PMID: 29220294 Free PMC article. Review.
-
Lipomatous tumors.Monogr Pathol. 1996;38:207-39. Monogr Pathol. 1996. PMID: 8744279 Review.
Cited by
-
Phosphorylation of IWS1 by AKT maintains liposarcoma tumor heterogeneity through preservation of cancer stem cell phenotypes and mesenchymal-epithelial plasticity.Oncogenesis. 2023 May 26;12(1):30. doi: 10.1038/s41389-023-00469-z. Oncogenesis. 2023. PMID: 37237004 Free PMC article.
-
Characterization of cancer stem-like cells derived from mouse induced pluripotent stem cells transformed by tumor-derived extracellular vesicles.J Cancer. 2014 Jul 5;5(7):572-84. doi: 10.7150/jca.8865. eCollection 2014. J Cancer. 2014. PMID: 25057308 Free PMC article.
-
Sex Differences and Bone Metastases of Breast, Lung, and Prostate Cancers: Do Bone Homing Cancers Favor Feminized Bone Marrow?Front Oncol. 2017 Aug 7;7:163. doi: 10.3389/fonc.2017.00163. eCollection 2017. Front Oncol. 2017. PMID: 28824875 Free PMC article. Review.
-
Cytokine signaling regulating adipose stromal cell trafficking.Adipocyte. 2016 Aug 12;5(4):369-374. doi: 10.1080/21623945.2016.1220452. eCollection 2016 Oct-Dec. Adipocyte. 2016. PMID: 27994950 Free PMC article.
-
Fatty acid mobilization from adipose tissue is mediated by CD36 posttranslational modifications and intracellular trafficking.JCI Insight. 2021 Sep 8;6(17):e147057. doi: 10.1172/jci.insight.147057. JCI Insight. 2021. PMID: 34314388 Free PMC article.
References
-
- Anaya DA, Lahat G, Wang X, Xiao L, Tuvin D, Pisters PW, Lev DC, Pollock RE. Establishing prognosis in retroperitoneal sarcoma: a new histology-based paradigm. Ann Surg Oncol. 2009;16:667–675. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

