L-amino acid ligase from Pseudomonas syringae producing tabtoxin can be used for enzymatic synthesis of various functional peptides
- PMID: 23770908
- PMCID: PMC3754701
- DOI: 10.1128/AEM.01003-13
L-amino acid ligase from Pseudomonas syringae producing tabtoxin can be used for enzymatic synthesis of various functional peptides
Abstract
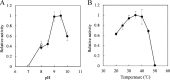
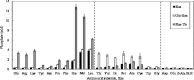
Functional peptides are expected to be beneficial compounds that improve our quality of life. To address the growing need for functional peptides, we have examined peptide synthesis by using microbial enzymes. l-Amino acid ligase (Lal) catalyzes the condensation of unprotected amino acids in an ATP-dependent manner and is applicable to fermentative production. Hence, Lal is a promising enzyme to achieve cost-effective synthesis. To obtain a Lal with novel substrate specificity, we focused on the putative Lal involved in the biosynthesis of the dipeptidic phytotoxin designated tabtoxin. The tabS gene was cloned from Pseudomonas syringae NBRC14081 and overexpressed in Escherichia coli cells. The recombinant TabS protein produced showed the broadest substrate specificity of any known Lal; it detected 136 of 231 combinations of amino acid substrates when dipeptide synthesis was examined. In addition, some new substrate specificities were identified and unusual amino acids, e.g., l-pipecolic acid, hydroxy-l-proline, and β-alanine, were found to be acceptable substrates. Furthermore, kinetic analysis and monitoring of the reactions over a short time revealed that TabS showed distinct substrate selectivity at the N and C termini, which made it possible to specifically synthesize a peptide without by-products such as homopeptides and heteropeptides with the reverse sequence. TabS specifically synthesized the following functional peptides, including their precursors: l-arginyl-l-phenylalanine (antihypertensive effect; yield, 62%), l-leucyl-l-isoleucine (antidepressive effect; yield, 77%), l-glutaminyl-l-tryptophan (precursor of l-glutamyl-l-tryptophan, which has antiangiogenic activity; yield, 54%), l-leucyl-l-serine (enhances saltiness; yield, 83%), and l-glutaminyl-l-threonine (precursor of l-glutamyl-l-threonine, which enhances saltiness; yield, 96%). Furthermore, our results also provide new insights into tabtoxin biosynthesis.
Figures

References
-
- Santos S, Torcato I, Castanho MA. 2012. Biomedical applications of dipeptides and tripeptides. Biopolymers 98:288–293 - PubMed
-
- Mills S, Stanton C, Hill C, Ross RP. 2011. New developments and applications of bacteriocins and peptides in foods. Annu. Rev. Food Sci. Technol. 2:299–329 - PubMed
-
- Yagasaki M, Hashimoto S. 2008. Synthesis and application of dipeptides; current status and perspectives. Appl. Microbiol. Biotechnol. 81:13–22 - PubMed
-
- Kagebayashi T, Kontani N, Yamada Y, Mizushige T, Arai T, Kino K, Ohinata K. 2012. Novel CCK-dependent vasorelaxing dipeptide, Arg-Phe, decreases blood pressure and food intake in rodents. Mol. Nutr. Food Res. 56:1456–1463 - PubMed
-
- Hernández-Ledesma B, del Mar Contreras M, Recio I. 2011. Antihypertensive peptides: production, bioavailability and incorporation into foods. Adv. Colloid Interface Sci. 165:23–35 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

