Antiviral and anti-inflammatory activity of arbidol hydrochloride in influenza A (H1N1) virus infection
- PMID: 23770981
- PMCID: PMC4003028
- DOI: 10.1038/aps.2013.54
Antiviral and anti-inflammatory activity of arbidol hydrochloride in influenza A (H1N1) virus infection
Abstract
Aim: To investigate the effects of arbidol hydrochloride (ARB), a widely used antiviral agent, on the inflammation induced by influenza virus.
Methods: MDCK cells were infected with seasonal influenza A/FM/1/47 (H1N1) or pandemic influenza A/Hubei/71/2009 (H1N1). In vitro cytotoxicity and antiviral activity of ARB was determined using MTT assay. BALB/c mice were infected with A/FM/1/47 (H1N1). Four hours later the mice were administered ARB (45, 90, and 180 mg·kg(-1)·d(-1)) or the neuraminidase inhibitor oseltamivir (22.5 mg·kg(-1)·d(-1)) via oral gavage once a day for 5 d. Body-weight, median survival time, viral titer, and lung index of the mice were measured. The levels of inflammatory cytokines were examined using real-time RT-PCR and ELISA.
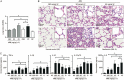
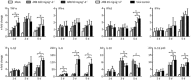
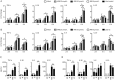
Results: Both H1N1 stains were equally sensitive to ARB as tested in vitro. In the infected mice, ARB (90 and 180 mg·kg(-1)·d(-1)) significantly decreased the mortality, alleviated virus-induced lung lesions and viral titers. Furthermore, ARB suppressed the levels of IL-1β, IL-6, IL-12, and TNF-α, and elevated the level of IL-10 in the bronchoalveolar lavage fluids and lung tissues. However, ARB did not significantly affect the levels of IFN-α and IFN-γ, but reduced the level of IFN-β1 in lung tissues at 5 dpi. In peritoneal macrophages challenged with A/FM/1/47 (H1N1) or poly I:C, ARB (20 μmol/L) suppressed the levels of IL-1β, IL-6, IL-12, and TNF-α, and elevated the level of IL-10. Oseltamivir produced comparable alleviation of virus-induced lung lesions with more reduction in the viral titers, but less effective modulation of the inflammatory cytokines.
Conclusion: ARB efficiently inhibits both H1N1 stains and diminishes both viral replication and acute inflammation through modulating the expression of inflammatory cytokines.
Figures

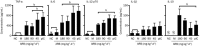
Similar articles
-
Inhibition of the infectivity and inflammatory response of influenza virus by Arbidol hydrochloride in vitro and in vivo (mice and ferret).Biomed Pharmacother. 2017 Jul;91:393-401. doi: 10.1016/j.biopha.2017.04.091. Epub 2017 May 2. Biomed Pharmacother. 2017. PMID: 28475918
-
Geniposide demonstrates anti-inflammatory and antiviral activity against pandemic A/Jiangsu/1/2009 (H1N1) influenza virus infection in vitro and in vivo.Antivir Ther. 2017;22(7):599-611. doi: 10.3851/IMP3152. Epub 2017 Mar 8. Antivir Ther. 2017. PMID: 28272019
-
Sangju Cold Granule exerts anti-viral and anti-inflammatory activities against influenza A virus in vitro and in vivo.J Ethnopharmacol. 2024 Nov 15;334:118521. doi: 10.1016/j.jep.2024.118521. Epub 2024 Jul 3. J Ethnopharmacol. 2024. PMID: 38969152
-
Arbidol: The current demand, strategies, and antiviral mechanisms.Immun Inflamm Dis. 2023 Aug;11(8):e984. doi: 10.1002/iid3.984. Immun Inflamm Dis. 2023. PMID: 37647451 Free PMC article. Review.
-
The cytokine storms of COVID-19, H1N1 influenza, CRS and MAS compared. Can one sized treatment fit all?Cytokine. 2021 Aug;144:155593. doi: 10.1016/j.cyto.2021.155593. Epub 2021 May 26. Cytokine. 2021. PMID: 34074585 Free PMC article. Review.
Cited by
-
Antiviral potential of diosmin against influenza A virus.Sci Rep. 2025 May 17;15(1):17192. doi: 10.1038/s41598-025-00744-6. Sci Rep. 2025. PMID: 40382364 Free PMC article.
-
Cytokine Storm in COVID-19-Immunopathological Mechanisms, Clinical Considerations, and Therapeutic Approaches: The REPROGRAM Consortium Position Paper.Front Immunol. 2020 Jul 10;11:1648. doi: 10.3389/fimmu.2020.01648. eCollection 2020. Front Immunol. 2020. PMID: 32754159 Free PMC article.
-
Understanding Immune Responses to Viruses-Do Underlying Th1/Th2 Cell Biases Predict Outcome?Viruses. 2022 Jul 8;14(7):1493. doi: 10.3390/v14071493. Viruses. 2022. PMID: 35891472 Free PMC article. Review.
-
A molecular docking study revealed that synthetic peptides induced conformational changes in the structure of SARS-CoV-2 spike glycoprotein, disrupting the interaction with human ACE2 receptor.Int J Biol Macromol. 2020 Dec 1;164:66-76. doi: 10.1016/j.ijbiomac.2020.07.174. Epub 2020 Jul 18. Int J Biol Macromol. 2020. PMID: 32693122 Free PMC article.
-
Arbidol as a broad-spectrum antiviral: an update.Antiviral Res. 2014 Jul;107:84-94. doi: 10.1016/j.antiviral.2014.04.006. Epub 2014 Apr 24. Antiviral Res. 2014. PMID: 24769245 Free PMC article. Review.
References
-
- Fields BN, Knipe DM, Howley PM.Fields virology. 5th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007
-
- La Gruta NL, Kedzierska K, Stambas J, Doherty PC. A question of self-preservation: immunopathology in influenza virus infection. Immunol Cell Biol. 2007;85:85–92. - PubMed
-
- Tumpey TM, Basler CF, Aguilar PV, Zeng H, Solorzano A, Swayne DE, et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005;310:77–80. - PubMed
-
- CDC. Update: drug susceptibility of swine-origin influenza A (H1N1) viruses, April 2009 MMWR Morb Mortal Wkly Rep 200958433–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

