Effect of an oxygen-tolerant bifurcating butyryl coenzyme A dehydrogenase/electron-transferring flavoprotein complex from Clostridium difficile on butyrate production in Escherichia coli
- PMID: 23772070
- PMCID: PMC3754583
- DOI: 10.1128/JB.00321-13
Effect of an oxygen-tolerant bifurcating butyryl coenzyme A dehydrogenase/electron-transferring flavoprotein complex from Clostridium difficile on butyrate production in Escherichia coli
Abstract
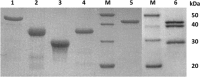
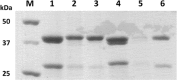
The butyrogenic genes from Clostridium difficile DSM 1296(T) have been cloned and expressed in Escherichia coli. The enzymes acetyl-coenzyme A (CoA) C-acetyltransferase, 3-hydroxybutyryl-CoA dehydrogenase, crotonase, phosphate butyryltransferase, and butyrate kinase and the butyryl-CoA dehydrogenase complex composed of the dehydrogenase and two electron-transferring flavoprotein subunits were individually produced in E. coli and kinetically characterized in vitro. While most of these enzymes were measured using well-established test systems, novel methods to determine butyrate kinase and butyryl-CoA dehydrogenase activities with respect to physiological function were developed. Subsequently, the individual genes were combined to form a single plasmid-encoded operon in a plasmid vector, which was successfully used to confer butyrate-forming capability to the host. In vitro and in vivo studies demonstrated that C. difficile possesses a bifurcating butyryl-CoA dehydrogenase which catalyzes the NADH-dependent reduction of ferredoxin coupled to the reduction of crotonyl-CoA also by NADH. Since the reoxidation of ferredoxin by a membrane-bound ferredoxin:NAD(+)-oxidoreductase enables electron transport phosphorylation, additional ATP is formed. The butyryl-CoA dehydrogenase from C. difficile is oxygen stable and apparently uses oxygen as a co-oxidant of NADH in the presence of air. These properties suggest that this enzyme complex might be well suited to provide butyryl-CoA for solventogenesis in recombinant strains. The central role of bifurcating butyryl-CoA dehydrogenases and membrane-bound ferredoxin:NAD oxidoreductases (Rhodobacter nitrogen fixation [RNF]), which affect the energy yield of butyrate fermentation in the clostridial metabolism, is discussed.
Figures


References
-
- Nölling J, Breton G, Omelchenko MV, Makarova KS, Zeng Q, Gibson R, Lee HM, Dubois J, Qiu D, Hitti J, Wolf YI, Tatusov RL, Sabathe F, Doucette-Stamm L, Soucaille P, Daly MJ, Bennett GN, Koonin EV, Smith DR. 2001. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J. Bacteriol. 183: 4823– 4838 - PMC - PubMed
-
- Sebaihia M, Peck MW, Minton NP, Thomson NR, Holden MT, Mitchell WJ, Carter AT, Bentley SD, Mason DR, Crossman L, Paul CJ, Ivens A, Wells-Bennik MH, Davis IJ, Cerdeno-Tarraga AM, Churcher C, Quail MA, Chillingworth T, Feltwell T, Fraser A, Goodhead I, Hance Z, Jagels K, Larke N, Maddison M, Moule S, Mungall K, Norbertczak H, Rabbinowitsch E, Sanders M, Simmonds M, White B, Whithead S, Parkhill J. 2007. Genome sequence of a proteolytic (group I) Clostridium botulinum strain Hall A and comparative analysis of the clostridial genomes. Genome Res. 17: 1082– 1092 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

