Isothermal titration calorimetry with micelles: Thermodynamics of inhibitor binding to carnitine palmitoyltransferase 2 membrane protein
- PMID: 23772395
- PMCID: PMC3668529
- DOI: 10.1016/j.fob.2013.04.003
Isothermal titration calorimetry with micelles: Thermodynamics of inhibitor binding to carnitine palmitoyltransferase 2 membrane protein
Abstract
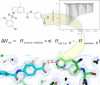
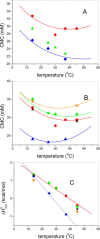
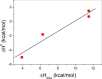
Carnitine palmitoyl transferase 2 (CPT-2) is a key enzyme in the mitochondrial fatty acid metabolism. The active site is comprised of a Y-shaped tunnel with distinct binding sites for the substrate acylcarnitine and the cofactor CoA. We investigated the thermodynamics of binding of four inhibitors directed against either the CoA or the acylcarnitine binding sites using isothermal titration calorimetry (ITC). CPT-2 is a monotopic membrane protein and was solubilized by β-octylglucoside (β-OG) above its critical micellar concentration (CMC) to perform inhibitor titrations in solutions containing detergent micelles. The CMC of β-OG in the presence of inhibitors was measured with ITC and small variations were observed. The inhibitors bound to rat CPT-2 (rCPT-2) with 1:1 stoichiometry and the dissociation constants were in the range of K D = 2-20 μM. New X-ray structures and docking models of rCPT-2 in complex with inhibitors enable an analysis of the thermodynamic data in the context of the interaction observed for the individual binding sites of the ligands. For all ligands the binding enthalpy was exothermic, and enthalpy as well as entropy contributed to the binding reaction, with the exception of ST1326 for which binding was solely enthalpy-driven. The substrate analog ST1326 binds to the acylcarnitine binding site and a heat capacity change close to zero suggests a balance of electrostatic and hydrophobic interactions. An excellent correlation of the thermodynamic (ITC) and structural (X-ray crystallography, models) data was observed suggesting that ITC measurements provide valuable information for optimizing inhibitor binding in drug discovery.
Keywords: CMC (critical micellar concentration); ITC (isothermal titration calorimetry); rCPT-2 (rat carnitine-palmitoyltransferase); β-OG (n-octyl-β-D-glucopyranoside).
Figures



References
-
- de Vries Y., Arvidson D.N., Waterham H.R., Cregg J.M., Woldegiorgis G. Functional characterization of mitochondrial carnitine palmitoyltransferases I and II expressed in the yeast Pichia pastoris. Biochemistry. 1997;36:5285–5292. - PubMed
-
- Bonnefont J.P., Djouadi F., Prip-Buus C., Gobin S., Munnich A., Bastin J. Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol. Aspects Med. 2004;25:495–520. - PubMed
-
- Zammit V.A. Carnitine acyltransferases: functional significance of subcellular distribution and membrane topology. Prog. Lipid Res. 1999;38:199–224. - PubMed
-
- Foster D.W. The role of the carnitine system in human metabolism. Ann. N.Y. Acad. Sci. 2004;1033:1–16. - PubMed
-
- Deschauer M., Wieser T., Zierz S. Muscle carnitine palmitoyltransferase II deficiency: clinical and molecular genetic features and diagnostic aspects. Arch. Neurol. 2005;62:37–41. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

