Musculoskeletal regeneration and its implications for the treatment of tendinopathy
- PMID: 23772908
- PMCID: PMC3721460
- DOI: 10.1111/iep.12031
Musculoskeletal regeneration and its implications for the treatment of tendinopathy
Abstract
Tendinopathies are common muskoloskeletal injuries that lead to pain and disability. Development and pathogenesis of tendinopathy is attributed to progressive pathological changes to the structure, function, and biology of tendon. The nature of this disease state, whether acquired by acute or chronic injury, is being actively investigated. Scarring, disorganized tissue, and loss of function characterize adult tendon healing. Recent work from animal models has begun to reveal the potential for adult mammalian tendon regeneration, the replacement of diseased with innate tissue. This review discusses what is known about musculoskeletal regeneration from a molecular perspective and how these findings can be applied to tendinopathy. Non-mammalian and mammalian models are discussed with emphasis on the potential of Murphy Roths Large mice to serve as a model of adult tendon regeneration. Comparison of regeneration in non-mammals, foetal mammals and adult mammals emphasizes distinctly different contributing factors to effective regeneration.
Keywords: C57BL/6J; MRL; MRL/MpJ; healing; regeneration; scar-mediated; scarless; tendon.
© 2013 The Authors. International Journal of Experimental Pathology © 2013 International Journal of Experimental Pathology.
Figures

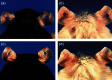



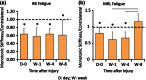
References
-
- Abdullah I, Lepore JJ, Epstein JA, Parmacek MS, Gruber PJ. MRL mice fail to heal the heart in response to ischemia-reperfusion injury. Wound Repair Regen. 2005;13:205–208. - PubMed
-
- Abshagen K, Eipel C, Vollmar B. A critical appraisal of the hemodynamic signal driving liver regeneration. Langenbecks Arch. Surg. 2012;397:579–590. - PubMed
-
- Adzick NS, Harrison MR, Glick PL, et al. Comparison of fetal, newborn, and adult wound healing by histologic, enzyme-histochemical, and hydroxyproline determinations. J. Pediatr. Surg. 1985;20:315–319. - PubMed
-
- Alibardi L. Morphological and cellular aspects of tail and limb regeneration in lizards. A model system with implications for tissue regeneration in mammals. Adv. Anat. Embryol. Cell Biol. 2010;207:iii. v–x, 1–109. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

