Experimental support for a desolvation energy term in governing equations for binding equilibria
- PMID: 23773139
- PMCID: PMC3754792
- DOI: 10.1021/jp402632a
Experimental support for a desolvation energy term in governing equations for binding equilibria
Abstract
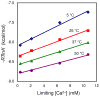
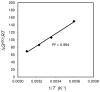
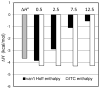
This study introduces a new thermodynamic framework for aqueous reaction equilibria that treats water as a coreactant in the development of a general binding equation. The approach features an explicit consideration for the change in hydration that occurs when two solvated surfaces come into contact. As an outcome of this framework, the standard-state free energy of binding is defined by the summation of two terms: the traditional term (-RT ln Ki) plus a desolvation free-energy term that is weighted by the number of complexes formed at equilibrium. The new formalism suggests that the equilibrium ratio, Ki, is not a constant and that the observed concentration dependence of Ki may be used to obtain the molar desolvation energy and the standard-state free energy at infinite dilution. The governing equation is supported by results from isothermal titration calorimetry using the chelation of calcium(II) by EDTA as a model binding reaction. This work may have far-reaching implications for solution thermodynamics, including an explanation for the oft-noted discrepancy between the enthalpy values obtained by calorimetry and those from the van't Hoff approach.
Figures

References
-
- Frank HS, Evans MW. Free Volume and Entropy in Condensed Systems. III. Entropy in Binary Liquid Mixtures; Partial Molal Entropy in Dilute Solutions; Structure and Thermodynamics in Aqueous Electrolytes. J Chem Phys. 1945;13:507–532.
-
- Dill KA. Dominant Forces in Protein Folding. Biochemistry. 1990;29:7133–7155. - PubMed
-
- Wilson EK. Water’s Role in Drug Discovery. Chem Eng News. 2012;90:64–65.
-
- Ball P. Biophysics: More Than a Bystander. Nature. 2011;478:467–468. - PubMed
-
- McNaught AD, Wilkinson A, editors. IUPAC. Compendium of Chemical Terminology. 2. Blackwell Scientific Publications; Oxford: 1997. (The “Gold Book”)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

