Cost-effectiveness of Haemophilus influenzae type b conjugate vaccine in low- and middle-income countries: regional analysis and assessment of major determinants
- PMID: 23773595
- PMCID: PMC5749634
- DOI: 10.1016/j.jpeds.2013.03.031
Cost-effectiveness of Haemophilus influenzae type b conjugate vaccine in low- and middle-income countries: regional analysis and assessment of major determinants
Abstract
Objectives: To estimate the cost-effectiveness of Haemophilus influenzae type b (Hib) conjugate vaccine in low- and middle-income countries and identify the model variables, which are most important for the result.
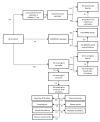
Study design: A static decision tree model was developed to predict incremental costs and health impacts. Estimates were generated for 4 country groups: countries eligible for funding by the GAVI Alliance in Africa and Asia, lower middle-income countries, and upper middle-income countries. Values, including disease incidence, case fatality rates, and treatment costs, were based on international country estimates and the scientific literature.
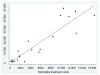
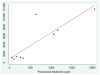
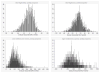
Results: From the societal perspective, it is estimated that the probability of Hib conjugate vaccine cost saving is 34%-53% in Global Alliance for Vaccines and Immunization eligible African and Asian countries, respectively. In middle-income countries, costs per discounted disability adjusted life year averted are between US$37 and US$733. Variation in vaccine prices and risks of meningitis sequelae and mortality explain most of the difference in results. For all country groups, disease incidence cause the largest part of the uncertainty in the result.
Conclusions: Hib conjugate vaccine is cost saving or highly cost-effective in low- and middle-income settings. This conclusion is especially influenced by the recent decline in Hib conjugate vaccine prices and new data revealing the high costs of lost productivity associated with meningitis sequelae.
Copyright © 2013. Published by Mosby, Inc.
Conflict of interest statement
The authors declare no conflicts of interest, real or perceived.
Figures
References
-
- Wenger JD. Epidemiology of Haemophilus influenzae type b disease and impact of Haemophilus influenzae type b conjugate vaccines in the United States and Canada. Pediatr Infect Dis J. 1998;17:S132–6. - PubMed
-
- Ojo LR, O’Loughlin RE, Cohen AL, Loo JD, Edmond KM, Shetty SS, et al. Global use of Haemophilus influenzae type b conjugate vaccine. Vaccine. 2010;28:7117–22. - PubMed
-
- International Vaccine Access Center (IVAC) VIMS Report: Global Vaccine Introduction. Johns Hopkins Bloomberg School of Public Health; Aug, 2012. [Accessed September 11, 2012]. Available at: http://wwwjhsphedu/research/centers-and-institutes/ivac/vims/IVAC-VIMS_R....
-
- Watt JP, Wolfson LJ, O’Brien KL, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet. 2009;374:903–11. - PubMed
-
- WHO. WHO guide for standardization of economic evaluations of immunization programmes. Immunization, Vaccines and Biologicals Department, World Health Organization WHO/IVB/08.14; Geneva: 2008.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

