Differentiating the roles of STAT5B and STAT5A in human CD4+ T cells
- PMID: 23773921
- PMCID: PMC4169138
- DOI: 10.1016/j.clim.2013.04.014
Differentiating the roles of STAT5B and STAT5A in human CD4+ T cells
Abstract
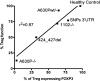
STAT5A and STAT5B are highly homologous proteins whose distinctive roles in human immunity remain unclear. However, STAT5A sufficiency cannot compensate for STAT5B defects, and human STAT5B deficiency, a rare autosomal recessive primary immunodeficiency, is characterized by chronic lung disease, growth failure and autoimmunity associated with regulatory T cell (Treg) reduction. We therefore hypothesized that STAT5A and STAT5B play unique roles in CD4(+) T cells. Upon knocking down STAT5A or STAT5B in human primary T cells, we found differentially regulated expression of FOXP3 and IL-2R in STAT5B knockdown T cells and down-regulated Bcl-X only in STAT5A knockdown T cells. Functional ex vivo studies in homozygous STAT5B-deficient patients showed reduced FOXP3 expression with impaired regulatory function of STAT5B-null Treg cells, also of increased memory phenotype. These results indicate that STAT5B and STAT5A act partly as non-redundant transcription factors and that STAT5B is more critical for Treg maintenance and function in humans.
Keywords: FOXP3; Regulatory T cells (Treg); STAT5; T cell development; T cell receptor excision circles; TREC; Treg; forkhead box P3; regulatory T cell.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
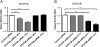


References
-
- Boucheron C, Dumon S, Santos SC, Moriggl R, Hennighausen L, Gisselbrecht S, Gouilleux F. A single amino acid in the DNA binding regions of STAT5A and STAT5B confers distinct DNA binding specificities. J. Biol. Chem. 1998;273:33936–33941. - PubMed
-
- Grimley PM, Dong F, Rui H. Stat5a and Stat5b: fraternal twins of signal transduction and transcriptional activation. Cytokine Growth Factor Rev. 1999;10:131–157. - PubMed
-
- Snow JW, Abraham N, Ma MC, Herndier BG, Pastuszak AW, Goldsmith MA. Loss of tolerance and autoimmunity affecting multiple organs in STAT5A/5B-deficient mice. J. Immunol. 2003;171:5042–5050. - PubMed
-
- Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

