Neural mechanisms of risky decision-making and reward response in adolescent onset cannabis use disorder
- PMID: 23773952
- PMCID: PMC3786021
- DOI: 10.1016/j.drugalcdep.2013.05.020
Neural mechanisms of risky decision-making and reward response in adolescent onset cannabis use disorder
Abstract
Background: Neural mechanisms of decision-making and reward response in adolescent cannabis use disorder (CUD) are underexplored.
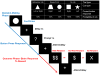
Methods: Three groups of male adolescents were studied: CUD in full remission (n=15); controls with psychopathology without substance use disorder history (n=23); and healthy controls (n=18). We investigated neural processing of decision-making and reward under conditions of varying risk and uncertainty with the Decision-Reward Uncertainty Task while participants were scanned using functional magnetic resonance imaging.
Results: Abstinent adolescents with CUD compared to controls with psychopathology showed hyperactivation in one cluster that spanned left superior parietal lobule/left lateral occipital cortex/precuneus while making risky decisions that involved uncertainty, and hypoactivation in left orbitofrontal cortex to rewarded outcomes compared to no-reward after making risky decisions. Post hoc region of interest analyses revealed that both control groups significantly differed from the CUD group (but not from each other) during both the decision-making and reward outcome phase of the Decision-Reward Uncertainty Task. In the CUD group, orbitofrontal activations to reward significantly and negatively correlated with total number of individual drug classes the CUD patients experimented with prior to treatment. CUD duration significantly and negatively correlated with orbitofrontal activations to no-reward.
Conclusions: The adolescent CUD group demonstrated distinctly different activation patterns during risky decision-making and reward processing (after risky decision-making) compared to both the controls with psychopathology and healthy control groups. These findings suggest that neural differences in risky decision-making and reward processes are present in adolescent addiction, persist after remission from first CUD treatment, and may contribute to vulnerability for adolescent addiction.
Keywords: Adolescence; Behavioral risk; Cannabis use disorder; Decision-making; Orbitofrontal cortex; Reward response.
Copyright © 2013 Elsevier Ireland Ltd. All rights reserved.
Figures




References
-
- Ameri A. The effects of cannabinoids on the brain. Progress in Neurobiology. 1999;58:315–348. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

