Contributions of the Kölliker-Fuse nucleus to coordination of breathing and swallowing
- PMID: 23774145
- PMCID: PMC4338573
- DOI: 10.1016/j.resp.2013.06.003
Contributions of the Kölliker-Fuse nucleus to coordination of breathing and swallowing
Abstract
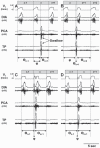
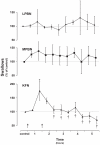
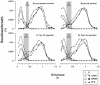
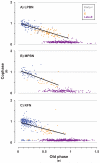
Herein we compare the effects of perturbations in the Kölliker-Fuse nucleus (KFN) and the lateral (LPBN) and medial (MPBN) parabrachial nuclei on the coordination of breathing and swallowing. Cannula was chronically implanted in goats through which ibotenic acid (IA) was injected while awake. Swallows in late expiration (E) always reset while swallows in early inspiration (I) never reset the respiratory rhythm. Before cannula implantation, all other E and I swallows did not reset the respiratory rhythm, and had small effects on E and I duration and tidal volume (VT). However, after cannula implantation in the MPBN and KFN, E and I swallows reset the respiratory rhythm and increased the effects on I and E duration and VT. Subsequent injection of IA into the KFN eliminated the respiratory phase resetting of swallows but exacerbated the effects on I and E duration and VT. We conclude that the KFN and to a lesser extent the MPBN contribute to coordination of breathing and swallowing.
Copyright © 2013. Published by Elsevier B.V.
Figures





References
-
- Bianchi AL, Denavit-Saiboe M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties and neurotransmitters. Physiological Reviews. 1995 Jan 1;75:1–45. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

