Multicenter study of high-dose daptomycin for treatment of enterococcal infections
- PMID: 23774437
- PMCID: PMC3754344
- DOI: 10.1128/AAC.00526-13
Multicenter study of high-dose daptomycin for treatment of enterococcal infections
Abstract
Enterococci are among the leading pathogens isolated in hospital-acquired infections. Current antimicrobial options for vancomycin-resistant enterococci (VRE) are limited. Prior data suggest that daptomycin at >6 mg/kg of body weight/day may be used to treat enterococcal infections. We retrospectively evaluated the effectiveness and safety of high-dose daptomycin (HD-daptomycin) therapy (>6 mg/kg) in a multicenter cohort of adult patients with enterococcal infections to describe the characteristics and outcomes. Two hundred forty-five patients were evaluated. Enterococcus faecium was identified in 175 (71%), followed by Enterococcus faecalis in 49 (20%) and Enterococcus spp. in 21 (9%); overall, 204 (83%) isolates were VRE. Enterococcal infections included bacteremia (173, 71%) and intra-abdominal (35, 14%) and bone and joint (25, 10%) infections. The median dosage and duration of HD-daptomycin were 8.2 mg/kg/day (interquartile range [IQR], 7.7 to 9.7) and 10 days (IQR, 6 to 15), respectively. The overall clinical success rate was 89% (193/218), and microbiological eradication was observed in 93% (177/191) of patients. The median time to clearance of blood cultures on HD-daptomycin was 3 days (IQR, 2 to 5). The 30-day all-cause mortality rate was 27%, and 5 (2%) patients developed daptomycin-nonsusceptible enterococcal strains while on HD-daptomycin. Seven patients (3%) had creatine phosphokinase (CPK) elevation, yet no HD-daptomycin regimen was discontinued due to an elevated CPK and all patients were asymptomatic. Overall, there was a high frequency of clinical success and microbiological eradication in patients treated with HD-daptomycin for enterococcal infections, even in patients with complicated and difficult-to-treat infections. No adverse event-related discontinuation of HD-daptomycin was noted. HD-daptomycin may be an option for the treatment of enterococcal infections.
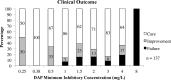
Figures


References
-
- Biedenbach DJ, Moet GJ, Jones RN. 2004. Occurrence and antimicrobial resistance pattern comparisons among bloodstream infection isolates from the SENTRY Antimicrobial Surveillance Program (1997–2002). Diagn. Microbiol. Infect. Dis. 50:59–69 - PubMed
-
- Carmeli Y, Eliopoulos G, Mozaffari E, Samore M. 2002. Health and economic outcomes of vancomycin-resistant enterococci. Arch. Intern. Med. 162:2223–2228 - PubMed
-
- Bhavnani SM, Drake JA, Forrest A, Deinhart JA, Jones RN, Biedenbach DJ, Ballow CH. 2000. A nationwide, multicenter, case-control study comparing risk factors, treatment, and outcome for vancomycin-resistant and -susceptible enterococcal bacteremia. Diagn. Microbiol. Infect. Dis. 36:145–158 - PubMed
-
- Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 29:996–1011 - PubMed
-
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309–317 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

