Fracture healing with alendronate treatment in the Brtl/+ mouse model of osteogenesis imperfecta
- PMID: 23774443
- PMCID: PMC3999166
- DOI: 10.1016/j.bone.2013.06.003
Fracture healing with alendronate treatment in the Brtl/+ mouse model of osteogenesis imperfecta
Abstract
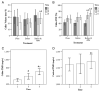
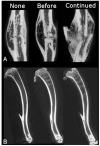
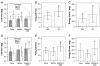
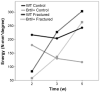
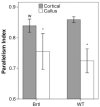
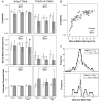
Osteogenesis imperfecta (OI) is a heritable bone dysplasia characterized by increased skeletal fragility. Patients are often treated with bisphosphonates to attempt to reduce fracture risk. However, bisphosphonates reside in the skeleton for many years and long-term administration may impact bone material quality. Acutely, there is concern about risk of non-union of fractures that occur near the time of bisphosphonate administration. This study investigated the effect of alendronate, a potent aminobisphosphonate, on fracture healing. Using the Brtl/+ murine model of type IV OI, tibial fractures were generated in 8-week-old mice that were untreated, treated with alendronate before fracture, or treated before and after fracture. After 2, 3, or 5 weeks of healing, tibiae were assessed using microcomputed tomography (μCT), torsion testing, quantitative histomorphometry, and Raman microspectroscopy. There were no morphologic, biomechanical or histomorphometric differences in callus between untreated mice and mice that received alendronate before fracture. Alendronate treatment before fracture did not cause a significant increase in cartilage retention in fracture callus. Both Brtl/+ and WT mice that received alendronate before and after fracture had increases in the callus volume, bone volume fraction and torque at failure after 5 weeks of healing. Raman microspectroscopy results did not show any effects of alendronate in wild-type mice, but calluses from Brtl/+ mice treated with alendronate during healing had a decreased mineral-to-matrix ratio, decreased crystallinity and an increased carbonate-to-phosphate ratio. Treatment with alendronate altered the dynamics of healing by preventing callus volume decreases later in the healing process. Fracture healing in Brtl/+ untreated animals was not significantly different from animals in which alendronate was halted at the time of fracture.
Keywords: Bisphosphonates; Bone; Fracture repair; Osteogenesis imperfecta; Rodent.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

References
-
- Marini JC, Forlino A, Cabral WA, Barnes AM, San Antonio JD, Milgrom S, et al. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum Mutat. 2007;28(3):209–21. - PMC - PubMed
-
- Marini JC, Cabral WA, Barnes AM, Chang W. Components of the collagen prolyl 3-hydroxylation complex are crucial for normal bone development. Cell Cycle. 2007;6(14):1675–81. - PubMed
-
- Herring JA, Tachdjian MO, editors. Tachdjian’s Pediatric Orthopaedics. 3rd ed Vol. 3. W.B. Saunders; Philadelphia: 2002.
-
- Cheung MS, Glorieux FH, Rauch F. Natural history of hyperplastic callus formation in osteogenesis imperfecta type V. J Bone Miner Res. 2007;22(8):1181–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

