Patient-reported outcomes (PROs): putting the patient perspective in patient-centered outcomes research
- PMID: 23774513
- PMCID: PMC3771694
- DOI: 10.1097/MLR.0b013e31829b1d84
Patient-reported outcomes (PROs): putting the patient perspective in patient-centered outcomes research
Abstract
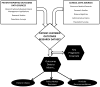
Patient-centered outcomes research (PCOR) aims to improve care quality and patient outcomes by providing information that patients, clinicians, and family members need regarding treatment alternatives, and emphasizing patient input to inform the research process. PCOR capitalizes on available data sources and generates new evidence to provide timely and relevant information and can be conducted using prospective data collection, disease registries, electronic medical records, aggregated results from prior research, and administrative claims. Given PCOR's emphasis on the patient perspective, methods to incorporate patient-reported outcomes (PROs) are critical. PROs are defined by the US Food and Drug Administration as "Any report coming directly from patients… about a health condition and its treatment." However, PROs have not routinely been collected in a way that facilitates their use in PCOR. Electronic medical records, disease registries, and administrative data have only rarely collected, or been linked to, PROs. Recent technological developments facilitate the electronic collection of PROs and linkage of PRO data, offering new opportunities for putting the patient perspective in PCOR. This paper describes the importance of and methods for using PROs for PCOR. We (1) define PROs; (2) identify how PROs can be used in PCOR and the critical role of electronic data methods for facilitating the use of PRO data in PCOR; (3) outline the challenges and key unanswered questions that need to be addressed for the routine use of PROs in PCOR; and (4) discuss policy and research interventions to accelerate the integration of PROs with clinical data.
Figures
References
-
- Agency for Healthcare Research and Quality. [Accessed December 10, 2012];What is Comparative Effectiveness Research. Available at: http://www.effectivehealthcare.ahrq.gov/index.cfm/what-is-comparative-ef...
-
- Patient Centered Outcomes Research Institute. [Accessed December 10, 2012]; Available at: http://www.pcori.org/
-
- U S. Food and Drug Administration. Guidance for Industry Patient Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Federal Register. 2009;74(35):65132–3.
-
- National Quality Forum. [Accessed December 10, 2012];Patient-Reported Outcomes. Available at: http://www.qualityforum.org/Projects/n-r/Patient-Reported_Outcomes/Patie....
-
- Acquadro C, Berzon R, Dubois D, et al. Incorporating the patient’s perspective into drug development and communication: an ad hoc task force report of the Patient-Reported Outcomes (PRO) Harmonization Group Meeting at the Food and Drug Administration, February 16, 2001. Value Health. 2003;6:522–31. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

