Production and release of antimicrobial and immune defense proteins by mammary epithelial cells following Streptococcus uberis infection of sheep
- PMID: 23774600
- PMCID: PMC3754230
- DOI: 10.1128/IAI.00291-13
Production and release of antimicrobial and immune defense proteins by mammary epithelial cells following Streptococcus uberis infection of sheep
Abstract
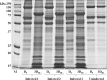
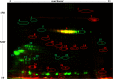
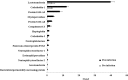
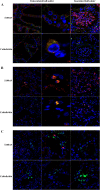
Investigating the innate immune response mediators released in milk has manifold implications, spanning from elucidation of the role played by mammary epithelial cells (MECs) in fighting microbial infections to the discovery of novel diagnostic markers for monitoring udder health in dairy animals. Here, we investigated the mammary gland response following a two-step experimental infection of lactating sheep with the mastitis-associated bacterium Streptococcus uberis. The establishment of infection was confirmed both clinically and by molecular methods, including PCR and fluorescent in situ hybridization of mammary tissues. Proteomic investigation of the milk fat globule (MFG), a complex vesicle released by lactating MECs, enabled detection of enrichment of several proteins involved in inflammation, chemotaxis of immune cells, and antimicrobial defense, including cathelicidins and calprotectin (S100A8/S100A9), in infected animals, suggesting the consistent involvement of MECs in the innate immune response to pathogens. The ability of MECs to produce and release antimicrobial and immune defense proteins was then demonstrated by immunohistochemistry and confocal immunomicroscopy of cathelicidin and the calprotectin subunit S100A9 on mammary tissues. The time course of their release in milk was also assessed by Western immunoblotting along the course of the experimental infection, revealing the rapid increase of these proteins in the MFG fraction in response to the presence of bacteria. Our results support an active role of MECs in the innate immune response of the mammary gland and provide new potential for the development of novel and more sensitive tools for monitoring mastitis in dairy animals.
Figures
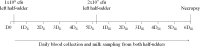




References
-
- De Vliegher S, Fox LK, Piepers S, McDougall S, Barkema HW. 2012. Mastitis in dairy heifers: nature of the disease, potential impact, prevention, and control. J. Dairy Sci. 95:1025–1040 - PubMed
-
- Marogna G, Rolesu S, Lollai S, Tola S, Leori S. 2010. Clinical findings in sheep farms affected by recurrent bacterial mastitis. Small Rumin. Res. 88:119–125
-
- Rainard P, Riollet C. 2006. Innate immunity of the bovine mammary gland. Vet. Res. 37:369–400 - PubMed
-
- Beutler B. 2004. Innate immunity: an overview. Mol. Immunol. 40:845–859 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

