Two distinct secretion systems facilitate tissue invasion by the rice blast fungus Magnaporthe oryzae
- PMID: 23774898
- PMCID: PMC3709508
- DOI: 10.1038/ncomms2996
Two distinct secretion systems facilitate tissue invasion by the rice blast fungus Magnaporthe oryzae
Abstract
To cause plant diseases, pathogenic micro-organisms secrete effector proteins into host tissue to suppress immunity and support pathogen growth. Bacterial pathogens have evolved several distinct secretion systems to target effector proteins, but whether fungi, which cause the major diseases of most crop species, also require different secretory mechanisms is not known. Here we report that the rice blast fungus Magnaporthe oryzae possesses two distinct secretion systems to target effectors during plant infection. Cytoplasmic effectors, which are delivered into host cells, preferentially accumulate in the biotrophic interfacial complex, a novel plant membrane-rich structure associated with invasive hyphae. We show that the biotrophic interfacial complex is associated with a novel form of secretion involving exocyst components and the Sso1 t-SNARE. By contrast, effectors that are secreted from invasive hyphae into the extracellular compartment follow the conventional secretory pathway. We conclude that the blast fungus has evolved distinct secretion systems to facilitate tissue invasion.
Figures
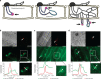
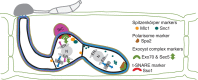
 ) were imaged in M. oryzae filamentous vegetative hyphae in vitro (a,b,d,f) and in tubular primary hyphae growing in rice cells (c,e,g). BICs (white arrows) were observed adjacent to the primary hyphal tips even without fluorescent labelling. (a) Spitzenkörper marker Mlc1:GFP (green) co-localizes (yellow) with the endocytic tracker dye FM4-64 (red) at tips of vegetative hyphae. This confocal image was acquired 10 min after FM4-64 addition. Images clockwise from upper left are bright-field; GFP; merged GFP and FM4-64; and FM4-64 alone. Similarly, Mlc1:GFP identifies Spitzenkörper in primary hyphae inside rice cells (Fig. 3a). (b–g) Images from left to right are bright-field; and GFP alone. (b) Polarisome component Spa2:GFP localizes to the tip of a vegetative hypha. (c) Polarisome component Spa2:GFP concentrates at the primary hyphal tip behind the BIC at 22 h.p.i. (d) v-SNARE GFP:Snc1 localizes at the vegetative hyphal tip. (e) v-SNARE GFP:Snc1 localizes in a bright fluorescent punctum near the primary hyphal tip at 24 h.p.i. (f) Exocyst component Exo70:GFP localizes at the vegetative hyphal tip. (g) Exocyst component Exo70:GFP localizes at the primary hyphal tip at 24 h.p.i. Scale bars, 5 μm.
) were imaged in M. oryzae filamentous vegetative hyphae in vitro (a,b,d,f) and in tubular primary hyphae growing in rice cells (c,e,g). BICs (white arrows) were observed adjacent to the primary hyphal tips even without fluorescent labelling. (a) Spitzenkörper marker Mlc1:GFP (green) co-localizes (yellow) with the endocytic tracker dye FM4-64 (red) at tips of vegetative hyphae. This confocal image was acquired 10 min after FM4-64 addition. Images clockwise from upper left are bright-field; GFP; merged GFP and FM4-64; and FM4-64 alone. Similarly, Mlc1:GFP identifies Spitzenkörper in primary hyphae inside rice cells (Fig. 3a). (b–g) Images from left to right are bright-field; and GFP alone. (b) Polarisome component Spa2:GFP localizes to the tip of a vegetative hypha. (c) Polarisome component Spa2:GFP concentrates at the primary hyphal tip behind the BIC at 22 h.p.i. (d) v-SNARE GFP:Snc1 localizes at the vegetative hyphal tip. (e) v-SNARE GFP:Snc1 localizes in a bright fluorescent punctum near the primary hyphal tip at 24 h.p.i. (f) Exocyst component Exo70:GFP localizes at the vegetative hyphal tip. (g) Exocyst component Exo70:GFP localizes at the primary hyphal tip at 24 h.p.i. Scale bars, 5 μm.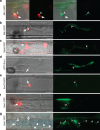
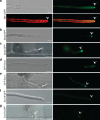
 ) labels the Spitzenkörper at the primary hyphal tip before hyphal differentiation at 24 h.p.i. Images left to right: merged bright-field, GFP and mRFP; merged GFP and mRFP; merged bright-field and GFP; and GFP alone. Scale bar, 5 μm. (b) After differentiation of bulbous IH at 27 h.p.i., Mlc1:GFP accumulates in a large fluorescent punctum in the BIC-associated bulbous IH cell (
) labels the Spitzenkörper at the primary hyphal tip before hyphal differentiation at 24 h.p.i. Images left to right: merged bright-field, GFP and mRFP; merged GFP and mRFP; merged bright-field and GFP; and GFP alone. Scale bar, 5 μm. (b) After differentiation of bulbous IH at 27 h.p.i., Mlc1:GFP accumulates in a large fluorescent punctum in the BIC-associated bulbous IH cell ( ) and near septa (*). Spitzenkörper-like fluorescence is not observed in growing bulbous IH tips. (c) Polarisome component Spa2:GFP localizes as a distinct punctum at the tip of growing IH (
) and near septa (*). Spitzenkörper-like fluorescence is not observed in growing bulbous IH tips. (c) Polarisome component Spa2:GFP localizes as a distinct punctum at the tip of growing IH ( ) at 27 h.p.i. Spa2 fluorescence is not observed in the subapical BIC-associated IH cell. In this image, saturated fluorescence from Pwl2:mRFP labels the rice cytoplasm and nucleus (*). This saturated Pwl2:mRFP fluorescence is seen in the EIHM compartment surrounding the BIC-associated cells, as previously reported. (d) v-SNARE GFP:Snc1 localizes to a large fluorescent punctum (
) at 27 h.p.i. Spa2 fluorescence is not observed in the subapical BIC-associated IH cell. In this image, saturated fluorescence from Pwl2:mRFP labels the rice cytoplasm and nucleus (*). This saturated Pwl2:mRFP fluorescence is seen in the EIHM compartment surrounding the BIC-associated cells, as previously reported. (d) v-SNARE GFP:Snc1 localizes to a large fluorescent punctum ( ) in the BIC-associated IH cell and to smaller vesicles in growing IH at 27 h.p.i. Images left to right: bright-field; and GFP alone. (e) Faint fluorescence from exocyst component Exo70:GFP can be observed in the subapical BIC-associated cell at 28 h.p.i. (f) t-SNARE Sso1:GFP (
) in the BIC-associated IH cell and to smaller vesicles in growing IH at 27 h.p.i. Images left to right: bright-field; and GFP alone. (e) Faint fluorescence from exocyst component Exo70:GFP can be observed in the subapical BIC-associated cell at 28 h.p.i. (f) t-SNARE Sso1:GFP ( ) localizes in the BIC-associated IH cell near the BIC in a first-invaded rice cell at 27 h.p.i. (g) t-SNARE Sso1:GFP (
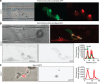
) localizes in the BIC-associated IH cell near the BIC in a first-invaded rice cell at 27 h.p.i. (g) t-SNARE Sso1:GFP ( ) in a second-invaded cell accumulated near BICs, as crescents at the tips of five primary IH (white arrows), and as puncta in two BIC-associated IH cells after differentiation (outline arrows). Shown at 40 h.p.i. (as described in Fig. 1a, right panel). Images left to right: bright-field; and GFP alone. Scale bars, 10 μm unless stated otherwise.
) in a second-invaded cell accumulated near BICs, as crescents at the tips of five primary IH (white arrows), and as puncta in two BIC-associated IH cells after differentiation (outline arrows). Shown at 40 h.p.i. (as described in Fig. 1a, right panel). Images left to right: bright-field; and GFP alone. Scale bars, 10 μm unless stated otherwise.


References
-
- Goldberg D. E. & Cowman A. F. Moving in and renovating: exporting proteins from Plasmodium into host erythrocytes. Nat. Rev. Microbiol. 8, 617–621 (2010). - PubMed
-
- Bozkurt T. O. Schornack S. Banfield M. J. & Kamoun S. Oomycetes, effectors, and all that jazz. Curr. Opin. Plant Biol. 15, 483–492 (2012). - PubMed
-
- Rafiqi M. Ellis J. G. Ludowici V. A. Hardham A. R. & Dodds P. N. Challenges and progress towards understanding the role of effectors in plant-fungal interactions. Curr. Opin. Plant Biol. 15, 477–482 (2012). - PubMed
-
- Alfano J. R. & Collmer A. Type III secretion system effector proteins: double agents in bacterial disease and plant defense. Annu. Rev. Phytopathol. 42, 385–414 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

