HMGN1 modulates nucleosome occupancy and DNase I hypersensitivity at the CpG island promoters of embryonic stem cells
- PMID: 23775126
- PMCID: PMC3753902
- DOI: 10.1128/MCB.00435-13
HMGN1 modulates nucleosome occupancy and DNase I hypersensitivity at the CpG island promoters of embryonic stem cells
Erratum in
-
Correction for Deng et al., "HMGN1 Modulates Nucleosome Occupancy and DNase I Hypersensitivity at the CpG Island Promoters of Embryonic Stem Cells".Mol Cell Biol. 2018 May 29;38(12):e00185-18. doi: 10.1128/MCB.00185-18. Print 2018 Jun 15. Mol Cell Biol. 2018. PMID: 29844108 Free PMC article. No abstract available.
Abstract
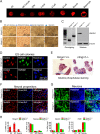
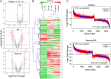
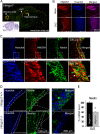
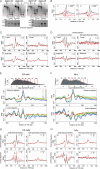
Chromatin structure plays a key role in regulating gene expression and embryonic differentiation; however, the factors that determine the organization of chromatin around regulatory sites are not fully known. Here we show that HMGN1, a nucleosome-binding protein ubiquitously expressed in vertebrate cells, preferentially binds to CpG island-containing promoters and affects the organization of nucleosomes, DNase I hypersensitivity, and the transcriptional profile of mouse embryonic stem cells and neural progenitors. Loss of HMGN1 alters the organization of an unstable nucleosome at transcription start sites, reduces the number of DNase I-hypersensitive sites genome wide, and decreases the number of nestin-positive neural progenitors in the subventricular zone (SVZ) region of mouse brain. Thus, architectural chromatin-binding proteins affect the transcription profile and chromatin structure during embryonic stem cell differentiation.
Figures


References
-
- Li M, Liu GH, Izpisua Belmonte JC. 2012. Navigating the epigenetic landscape of pluripotent stem cells. Nat. Rev. Mol. Cell Biol. 13:524–535 - PubMed
-
- Mattout A, Meshorer E. 2010. Chromatin plasticity and genome organization in pluripotent embryonic stem cells. Curr. Opin. Cell Biol. 22:334–341 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
