Sox9b is required for epicardium formation and plays a role in TCDD-induced heart malformation in zebrafish
- PMID: 23775563
- PMCID: PMC3876814
- DOI: 10.1124/mol.113.086413
Sox9b is required for epicardium formation and plays a role in TCDD-induced heart malformation in zebrafish
Abstract
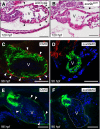
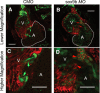
Activation of the transcription factor aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) prevents the formation of the epicardium and leads to severe heart malformations in developing zebrafish (Danio rerio). The downstream genes that cause heart malformation are not known. Because TCDD causes craniofacial malformations in zebrafish by downregulating the sox9b gene, we hypothesized that cardiotoxicity might also result from sox9b downregulation. We found that sox9b is expressed in the developing zebrafish heart ventricle and that TCDD exposure markedly reduces this expression. Furthermore, we found that manipulation of sox9b expression could phenocopy many but not all of the effects of TCDD at the heart. Loss of sox9b prevented the formation of epicardium progenitors comprising the proepicardium on the pericardial wall, and prevented the formation and migration of the epicardial layer around the heart. Zebrafish lacking sox9b showed pericardial edema, an elongated heart, and reduced blood circulation. Fish lacking sox9b failed to form valve cushions and leaflets. Sox9b is one of two mammalian Sox9 homologs, sox9b and sox9a. Knock down of sox9a expression did not cause cardiac malformations, or defects in epicardium development. We conclude that the decrease in sox9b expression in the heart caused by TCDD plays a role in many of the observed signs of cardiotoxicity. We find that while sox9b is expressed in myocardial cells, it is not normally expressed in the affected epicardial cells or progenitors. We therefore speculate that sox9b is involved in signals between the cardiomyocytes and the nascent epicardial cells.
Figures







References
-
- Andreasen EA, Spitsbergen JM, Tanguay RL, Stegeman JJ, Heideman W, Peterson RE. (2002) Tissue-specific expression of AHR2, ARNT2, and CYP1A in zebrafish embryos and larvae: effects of developmental stage and 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure. Toxicol Sci 68:403–419 - PubMed
-
- Antkiewicz DS, Burns CG, Carney SA, Peterson RE, Heideman W. (2005) Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol Sci 84:368–377 - PubMed
-
- Antkiewicz DS, Peterson RE, Heideman W. (2006) Blocking expression of AHR2 and ARNT1 in zebrafish larvae protects against cardiac toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci 94:175–182 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

