The winding pathway to erythropoietin along the chemistry-biology frontier: a success at last
- PMID: 23775885
- PMCID: PMC4729195
- DOI: 10.1002/anie.201301666
The winding pathway to erythropoietin along the chemistry-biology frontier: a success at last
Abstract
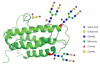
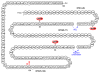
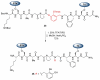
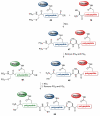
The total synthesis of a homogeneous erythropoietin (EPO), possessing the native amino acid sequence and chitobiose glycans at each of the three wild-type sites of N glycosylation, has been accomplished in our laboratory. We provide herein an account of our decade-long research effort en route to this formidable target compound. The optimization of the synergy of the two bedrock sciences we now call biology and chemistry was central to the success of the synthesis of EPO.
Keywords: amino acids; erythropoietin; glycoprotein; synthetic methods; total synthesis.
Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



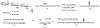



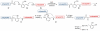





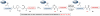





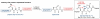
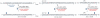



References
-
- Shin Y, Winans KA, Backes BJ, Kent SBH, Ellman JA, Bertozzi CR. J. Am. Chem. Soc. 1999;121:11684. For the first example of NCL in glycopeptide synthesis.
-
- Marcaurelle LA, Mizoue LS, Wilken J, Oldham L, Kent SBH, Handel TM, Bertozzi CR. Chem. Eur. J. 2001;7:1129. For the synthesis of alymphotactin, a glycosylated chemokine with a C-terminal mucin-like domain. - PubMed
-
- Yamamoto N, Tanabe Y, Okamoto R, Dawson PE, Kajihara Y. J. Am. Chem. Soc. 2008;130:501. For the synthesis of the 76-amino acid chemokine monocyte chemotactic protein-3. - PubMed
-
- Piontek C, Ring P, Harjes O, Heinlein C, Mezzato S, Lombana N, Pöhner C, Püttner M, Varón Silva D, Martin A, Schmid FX, Unverzagt C. Angew. Chem. 2009;121:1968. For the synthesis of ribonuclease C. - PubMed
- Angew. Chem. Int. Ed. 2009;48:1936.
- Piontek C, Silva DV, Heinlein C, Pöhner C, Mezzato S, Ring P, Martin A, Schmid FX, Unverzagt C. Angew. Chem. Int. Ed. 2009;48:1941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

