A randomized, masked, placebo-controlled study of darbepoetin alfa in preterm infants
- PMID: 23776118
- PMCID: PMC3691539
- DOI: 10.1542/peds.2013-0143
A randomized, masked, placebo-controlled study of darbepoetin alfa in preterm infants
Abstract
Background: A novel erythropoiesis stimulating agent (ESA), darbepoetin alfa (Darbe), increases hematocrit in anemic adults when administered every 1 to 3 weeks. Weekly Darbe dosing has not been evaluated in preterm infants. We hypothesized that infants would respond to Darbe by decreasing transfusion needs compared with placebo, with less-frequent dosing than erythropoietin (Epo).
Methods: Preterm infants 500 to 1250 g birth weight and ≤48 hours of age were randomized to Darbe (10 μg/kg, 1 time per week subcutaneously), Epo (400 U/kg, 3 times per week subcutaneously) or placebo (sham dosing) through 35 weeks' gestation. All received supplemental iron, folate, and vitamin E, and were transfused according to protocol. Transfusions (primary outcome), complete blood counts, absolute reticulocyte counts (ARCs), phlebotomy losses, and adverse events were recorded.
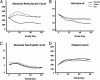
Results: A total of 102 infants (946 ± 196 g, 27.7 ± 1.8 weeks' gestation, 51 ± 25 hours of age at first dose) were enrolled. Infants in the Darbe and Epo groups received significantly fewer transfusions (P = .015) and were exposed to fewer donors (P = .044) than the placebo group (Darbe: 1.2 ± 2.4 transfusions and 0.7 ± 1.2 donors per infant; Epo: 1.2 ± 1.6 transfusions and 0.8 ± 1.0 donors per infant; placebo: 2.4 ± 2.9 transfusions and 1.2 ± 1.3 donors per infant). Hematocrit and ARC were higher in the Darbe and Epo groups compared with placebo (P = .001, Darbe and Epo versus placebo for both hematocrit and ARCs). Morbidities were similar among groups, including the incidence of retinopathy of prematurity.
Conclusions: Infants receiving Darbe or Epo received fewer transfusions and fewer donor exposures, and fewer injections were given to Darbe recipients. Darbepoetin and Epo successfully serve as adjuncts to transfusions in maintaining red cell mass in preterm infants.
Keywords: anemia; erythropoietin; prematurity; transfusions.
Figures
Comment in
-
Stimulating erythropoiesis in neonates.Am J Hematol. 2013 Nov;88(11):930-1. doi: 10.1002/ajh.23573. Epub 2013 Sep 19. Am J Hematol. 2013. PMID: 23963872 No abstract available.
References
-
- Bishara N, Ohls RK. Current controversies in the management of the anemia of prematurity. Semin Perinatol. 2009;33(1):29–34 - PubMed
-
- Maier RF, Obladen M, Müller-Hansen I, et al. European Multicenter Erythropoietin Beta Study Group . Early treatment with erythropoietin beta ameliorates anemia and reduces transfusion requirements in infants with birth weights below 1000 g. J Pediatr. 2002;141(1):8–15 - PubMed
-
- Warwood TL, Ohls RK, Wiedmeier SE, et al. . Single-dose darbepoetin administration to anemic preterm neonates. J Perinatol. 2005;25(11):725–730 - PubMed
-
- Warwood TL, Ohls RK, Lambert DK, et al. . Intravenous administration of darbepoetin to NICU patients. J Perinatol. 2006;26(5):296–300 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

