Implementation of timeline reforms speeds initiation of National Cancer Institute-sponsored trials
- PMID: 23776198
- PMCID: PMC3699438
- DOI: 10.1093/jnci/djt137
Implementation of timeline reforms speeds initiation of National Cancer Institute-sponsored trials
Abstract
Background: The National Cancer Institute (NCI) organized the Operational Efficiency Working Group in 2008 to develop recommendations for improving the speed with which NCI-sponsored clinical trials move from the idea stage to a protocol open to patient enrollment.
Methods: Given the many stakeholders involved, the Operational Efficiency Working Group advised a multifaceted approach to mobilize the entire research community to improve their business processes. New staff positions to monitor progress, protocol-tracking Web sites, and strategically planned conference calls were implemented. NCI staff and clinical teams at Cooperative Groups and Cancer Centers strived to achieve new target timelines but, most important, agreed to abide by absolute deadlines. For phase I-II studies and phase III studies, the target timelines are 7 months and 10 months, whereas the absolute deadlines were set at 18 and 24 months, respectively. Trials not activated by the absolute deadline are automatically disapproved.
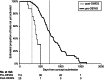
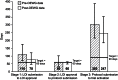
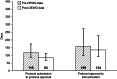
Results: The initial experience is encouraging and indicates a reduction in development times for phase I-II studies from the historical median of 541 days to a median of 442 days, an 18.3% decrease. The experience with phase III studies to date, although more limited (n = 25), demonstrates a 45.7% decrease in median days.
Conclusions: Based upon this progress, the NCI and the investigator community have agreed to reduce the absolute deadlines to 15 and 18 months for phase I-II and III trials, respectively. Emphasis on initiating trials rapidly is likely to help reduce the time it takes for clinical trial results to reach patients in need of new treatments.
Figures


References
-
- National Cancer Institute Report of the Clinical Trials Working Group of the National Cancer Advisory Board: Restructuring the National Cancer Clinical Trials Enterprise. 2005. http://ccct.cancer.gov/about/reports#clinical_trials Accessed May 13, 2013
-
- National Cancer Institute Report of the Operational Efficiency Working Group of the Clinical Trials and Translational Research Advisory Committee, Compressing the Timeline for Cancer Clinical Trial Activation, March 2010. http://ccct.cancer.gov/files/OEWG-Report Accessed May 13, 2013
Publication types
MeSH terms
Grants and funding
- HHSN261201100071C/CA/NCI NIH HHS/United States
- HHSN261201100100C/CA/NCI NIH HHS/United States
- U10CA032102/CA/NCI NIH HHS/United States
- U10CA025224/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
- U01CA062491/CA/NCI NIH HHS/United States
- U01CA099168/CA/NCI NIH HHS/United States
- U01CA062502/CA/NCI NIH HHS/United States
- U01CA132194/CA/NCI NIH HHS/United States
- U01CA069852/CA/NCI NIH HHS/United States
- U01 CA062502/CA/NCI NIH HHS/United States
- U10CA077202/CA/NCI NIH HHS/United States
- U01 CA062487/CA/NCI NIH HHS/United States
- U10CA098543/CA/NCI NIH HHS/United States
- U10CA076001/CA/NCI NIH HHS/United States
- HHSN261201100070C/CA/NCI NIH HHS/United States
- U10 CA077202/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA012027/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- HHSN261201100038C/CA/NCI NIH HHS/United States
- U01CA062490/CA/NCI NIH HHS/United States
- U10CA070095/CA/NCI NIH HHS/United States
- U10 CA027469/CA/NCI NIH HHS/United States
- U01CA062461/CA/NCI NIH HHS/United States
- U01CA069912/CA/NCI NIH HHS/United States
- U01CA062505/CA/NCI NIH HHS/United States
- HHSN261201100010C/CA/NCI NIH HHS/United States
- U01 CA062491/CA/NCI NIH HHS/United States
- U10CA031946/CA/NCI NIH HHS/United States
- U01 CA069912/CA/NCI NIH HHS/United States
- U01 CA099168/CA/NCI NIH HHS/United States
- HHSN261201100032C/CA/NCI NIH HHS/United States
- U01 CA062461/CA/NCI NIH HHS/United States
- HHSN261201100039C/CA/NCI NIH HHS/United States
- U01CA07657/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- U10CA080098/CA/NCI NIH HHS/United States
- U01 CA132194/CA/NCI NIH HHS/United States
- U10CA021115/CA/NCI NIH HHS/United States
- U01 CA062490/CA/NCI NIH HHS/United States
- U10 CA098543/CA/NCI NIH HHS/United States
- U10 CA021661/CA/NCI NIH HHS/United States
- U01CA132123/CA/NCI NIH HHS/United States
- U10 CA080098/CA/NCI NIH HHS/United States
- U10CA021661/CA/NCI NIH HHS/United States
- U01 CA069856/CA/NCI NIH HHS/United States
- U01CA062487/CA/NCI NIH HHS/United States
- U01 CA132123/CA/NCI NIH HHS/United States
- U10CA027469/CA/NCI NIH HHS/United States
- U10 CA076001/CA/NCI NIH HHS/United States
- UM1 CA186717/CA/NCI NIH HHS/United States
- U01 CA062505/CA/NCI NIH HHS/United States
- U01 CA069852/CA/NCI NIH HHS/United States
- HHSN261201100099C/CA/NCI NIH HHS/United States
- U10CA012027/CA/NCI NIH HHS/United States
- HHSN261201100010I/CA/NCI NIH HHS/United States
- U01CA69856/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

