B-cell maturation antigen is modified by a single N-glycan chain that modulates ligand binding and surface retention
- PMID: 23776238
- PMCID: PMC3704023
- DOI: 10.1073/pnas.1309417110
B-cell maturation antigen is modified by a single N-glycan chain that modulates ligand binding and surface retention
Abstract
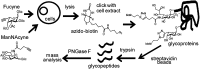
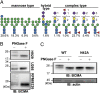
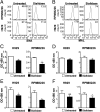
Glycosylation, an important posttranslational modification process, can modulate the structure and function of proteins, but its effect on the properties of plasma cells is largely unknown. In this study, we identified a panel of glycoproteins by click reaction with alkynyl sugar analogs in plasma cells coupled with mass spectrometry analysis. The B-cell maturation antigen (BCMA), an essential membrane protein for maintaining the survival of plasma cells, was identified as a glycoprotein exhibiting complex-type N-glycans at a single N-glycosylation site, asparagine 42. We then investigated the effect of N-glycosylation on the function of BCMA and found that the dexamethasone-induced apoptosis in malignant plasma cells can be rescued by treatment with BCMA ligands, such as a proliferation-inducing ligand (APRIL) and B-cell-activating factor (BAFF), whereas removal of terminal sialic acid on plasma cells further potentiated the ligand-mediated protection. This effect is associated with the increased surface retention of BCMA, leading to its elevated level on cell surface. In addition, the α1-3,-4 fucosylation, but not the terminal sialylation, assists the binding of BCMA with ligands in an in vitro binding assay. Together, our results highlight the importance of N-glycosylation on BCMA in the regulation of ligand binding and functions of plasma cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


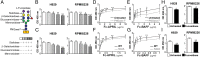
References
-
- Darce JR, Arendt BK, Wu X, Jelinek DF. Regulated expression of BAFF-binding receptors during human B cell differentiation. J Immunol. 2007;179(11):7276–7286. - PubMed
-
- Yang M, et al. B cell maturation antigen, the receptor for a proliferation-inducing ligand and B cell-activating factor of the TNF family, induces antigen presentation in B cells. J Immunol. 2005;175(5):2814–2824. - PubMed
-
- Mackay F, Ambrose C. The TNF family members BAFF and APRIL: The growing complexity. Cytokine Growth Factor Rev. 2003;14(3–4):311–324. - PubMed
-
- Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17(3):282–289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

