N-acetylglutamate synthase deficiency: an insight into the genetics, epidemiology, pathophysiology, and treatment
- PMID: 23776373
- PMCID: PMC3681184
- DOI: 10.2147/TACG.S12702
N-acetylglutamate synthase deficiency: an insight into the genetics, epidemiology, pathophysiology, and treatment
Abstract
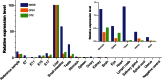
The conversion of ammonia into urea by the human liver requires the coordinated function of the 6 enzymes and 2 transporters of the urea cycle. The initial and rate-limiting enzyme of the urea cycle, carbamylphosphate synthetase 1 (CPS1), requires an allosteric activator, N-acetylglutamate (NAG). The formation of this unique cofactor from glutamate and acetyl Coenzyme-A is catalyzed by N-acetylglutamate synthase (NAGS). An absence of NAG as a consequence of NAGS deficiency may compromise flux through CPS1 and result in hyperammonemia. The NAGS gene encodes a 528-amino acid protein, consisting of a C-terminal catalytic domain, a variable segment, and an N-terminal mitochondrial targeting signal. Only 22 mutations in the NAGS gene have been reported to date, mostly in the catalytic domain. NAGS is primarily expressed in the liver and intestine. However, it is also surprisingly expressed in testis, stomach and spleen, and during early embryonic development at levels not concordant with the expression of other urea cycle enzymes, CPS1, or ornithine transcarbamylase. The purpose of NAGS expression in these tissues, and its significance to NAGS deficiency is as yet unknown. Inherited NAGS deficiency is the rarest of the urea cycle disorders, and we review the currently reported 34 cases. Treatment of NAGS deficiency with N-carbamyglutamate, a stable analog of NAG, can restore deficient urea cycle function and normalize blood ammonia in affected patients.
Keywords: N-acetyl-L-glutamate; N-acetylglutamate synthase; N-carbamyl-L-glutamate; hyperammonemia; urea cycle; urea cycle disorder.
Figures
References
-
- Brusilow SW, Maestri NE. Urea cycle disorders: diagnosis, pathophysiology, and therapy. Adv Pediatr. 1996;43:127–170. - PubMed
-
- Summar M. Current strategies for the management of neonatal urea cycle disorders. J Pediatr. 2001;138(Suppl 1):S30–S39. - PubMed
-
- Hall LM, Metzenberg RL, Cohen PP. Isolation and characterization of a naturally occurring cofactor of carbamyl phosphate biosynthesis. J Biol Chem. 1958;230(2):1013–1021. - PubMed
-
- Waterlow JC. The mysteries of nitrogen balance. Nutr Res Rev. 1999;12(1):25–54. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

