Ataxia-telangiectasia group D complementing gene (ATDC) promotes lung cancer cell proliferation by activating NF-κB pathway
- PMID: 23776433
- PMCID: PMC3680444
- DOI: 10.1371/journal.pone.0063676
Ataxia-telangiectasia group D complementing gene (ATDC) promotes lung cancer cell proliferation by activating NF-κB pathway
Abstract
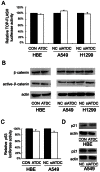
Previous studies suggested Ataxia-telangiectasia group D complementing gene (ATDC) as an oncogene in many types of cancer. However, its expression and biological functions in non-small cell lung cancer (NSCLC) remain unclear. Herein, we investigated its expression pattern in 109 cases of human NSCLC samples by immunohistochemistry and found that ATDC was overexpressed in 62 of 109 NSCLC samples (56.88%). ATDC overexpression correlated with histological type (p<0.0001), tumor status (p = 0.0227) and histological differentiation (p = 0.0002). Next, we overexpressed ATDC in normal human bronchial epithelial cell line HBE and depleted its expression in NSCLC cell lines A549 and H1299. MTT and colony formation assay showed that ATDC overexpression promoted cell proliferation while its depletion inhibited cell growth. Furthermore, cell cycle analysis showed that ATDC overexpression decreased the percentage of cells in G1 phase and increased the percentage of cells in S phase, while ATDC siRNA treatment increased the G1 phase percentage and decreased the S phase percentage. Further study revealed that ATDC overexpression could up-regulate cyclin D1 and c-Myc expression in HBE cells while its depletion down-regulated cyclin D1 and c-Myc expression in A549 and H1299 cells. In addition, ATDC overexpression was also associated with an increased proliferation index, cyclin D1 and c-Myc expression in human NSCLC samples. Further experiments demonstrated that ATDC up-regulated cyclin D1 and c-Myc expression independent of wnt/β-catenin or p53 signaling pathway. Interestingly, ATDC overexpression increased NF-κB reporter luciferase activity and p-IκB protein level. Correspondingly, NF-κB inhibitor blocked the effect of ATDC on up-regulation of cyclin D1 and c-Myc. In conclusion, we demonstrated that ATDC could promote lung cancer proliferation through NF-κB induced up-regulation of cyclin D1 and c-Myc.
Conflict of interest statement
Figures

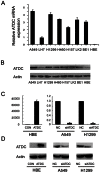

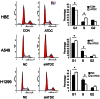
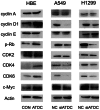
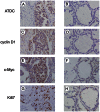

References
-
- Minna JD, Roth JA, Gazdar AF (2002) Focus on lung cancer. Cancer Cell 1: 49–52. - PubMed
-
- Jemal A, Siegel R, Xu J, Ward E Cancer statistics, 2010. CA Cancer J Clin 60: 277–300. - PubMed
-
- Poste G, Fidler IJ (1980) The pathogenesis of cancer metastasis. Nature 283: 139–146. - PubMed
-
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, et al. (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346: 92–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

