Role of NK cell subsets in organ-specific murine melanoma metastasis
- PMID: 23776508
- PMCID: PMC3679158
- DOI: 10.1371/journal.pone.0065599
Role of NK cell subsets in organ-specific murine melanoma metastasis
Abstract
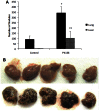
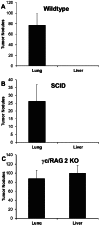
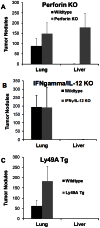
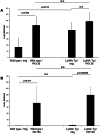
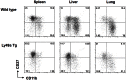
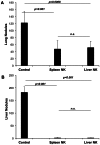
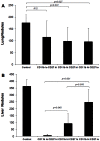
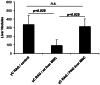
Tumor metastasis plays a major role in the morbidity and mortality of cancer patients. Among solid tumors that undergo metastasis, there is often a predilection to metastasize to a particular organ with, for example, prostate cancer preferentially metastasizing to bones and colon cancer preferentially metastasizing to the liver. Although many factors are thought to be important in establishing permissiveness for metastasis, the reasons for organ-specific predilection of each tumor are not understood. Using a B16 murine melanoma model, we tested the hypothesis that organ-specific NK cell subsets play a critical role in organ-specific metastasis of this tumor. Melanoma cells, given intravenously, readily colonized the lungs but not the liver. NK cell depletion (either iatrogenically or by using genetically targeted mice) resulted in substantial hepatic metastasis. Analysis of NK cell subsets, defined by the differential expression of a combination of CD27 and CD11b, indicated a significant difference in the distribution of NK cell subsets in the lung and liver with the mature subset being dominant in the lung and the immature subset being dominant in the liver. Several experimental approaches, including adoptive transfer, clearly indicated that the immature hepatic NK cell subset, CD27+ CD11b-, was protective against liver metastasis; this subset mediated its protection by a perforin-dependent cytotoxic mechanism. In contrast, the more mature NK cell subsets were more efficient at reducing pulmonary tumor load. These data indicate that organ-specific immune responses may play a pivotal role in determining the permissiveness of a given organ for the establishment of a metastatic niche.
Conflict of interest statement
Figures
References
-
- Coghlin C, Murray GI (2010) Current and emerging concepts in tumour metastasis. J Pathol 222: 1–15. - PubMed
-
- Guise T (2010) Examining the metastatic niche: targeting the microenvironment. Semin Oncol 37 Suppl 2S2–14. - PubMed
-
- Spano D, Zollo M (2012) Tumor microenvironment: a main actor in the metastasis process. Clin Exp Metastasis 29: 381–395. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

