Cholecystokinin-mediated RhoGDI phosphorylation via PKCα promotes both RhoA and Rac1 signaling
- PMID: 23776598
- PMCID: PMC3679036
- DOI: 10.1371/journal.pone.0066029
Cholecystokinin-mediated RhoGDI phosphorylation via PKCα promotes both RhoA and Rac1 signaling
Abstract
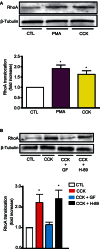
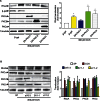
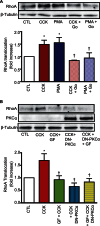
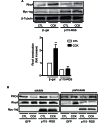
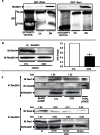
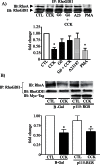
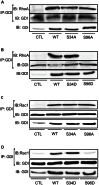
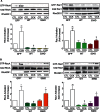
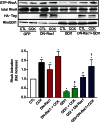
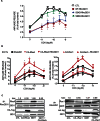
RhoA and Rac1 have been implicated in the mechanism of CCK-induced amylase secretion from pancreatic acini. In all cell types studied to date, inactive Rho GTPases are present in the cytosol bound to the guanine nucleotide dissociation inhibitor RhoGDI. Here, we identified the switch mechanism regulating RhoGDI1-Rho GTPase dissociation and RhoA translocation upon CCK stimulation in pancreatic acini. We found that both Gα13 and PKC, independently, regulate CCK-induced RhoA translocation and that the PKC isoform involved is PKCα. Both RhoGDI1 and RhoGDI3, but not RhoGDI2, are expressed in pancreatic acini. Cytosolic RhoA and Rac1 are associated with RhoGDI1, and CCK-stimulated PKCα activation releases the complex. Overexpression of RhoGDI1, by binding RhoA, inhibits its activation, and thereby, CCK-induced apical amylase secretion. RhoA translocation is also inhibited by RhoGDI1. Inactive Rac1 influences CCK-induced RhoA activation by preventing RhoGDI1 from binding RhoA. By mutational analysis we found that CCK-induced PKCα phosphorylation on RhoGDI1 at Ser96 releases RhoA and Rac1 from RhoGDI1 to facilitate Rho GTPases signaling.
Conflict of interest statement
Figures
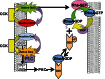
References
-
- Hori Y, Kikuchi A, Isomura M, Katayama M, Miura Y, et al. (1991) Post-translational modifications of the C-terminal region of the rho protein are important for its interaction with membranes and the stimulatory and inhibitory GDP/GTP exchange proteins. Oncogene 6: 515–522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

