Augmented BMPRIA-mediated BMP signaling in cranial neural crest lineage leads to cleft palate formation and delayed tooth differentiation
- PMID: 23776616
- PMCID: PMC3680418
- DOI: 10.1371/journal.pone.0066107
Augmented BMPRIA-mediated BMP signaling in cranial neural crest lineage leads to cleft palate formation and delayed tooth differentiation
Abstract
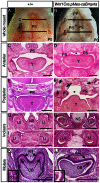
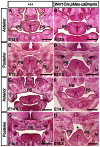
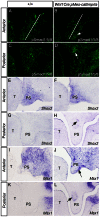
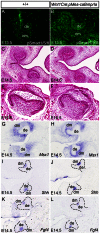
The importance of BMP receptor Ia (BMPRIa) mediated signaling in the development of craniofacial organs, including the tooth and palate, has been well illuminated in several mouse models of loss of function, and by its mutations associated with juvenile polyposis syndrome and facial defects in humans. In this study, we took a gain-of-function approach to further address the role of BMPR-IA-mediated signaling in the mesenchymal compartment during tooth and palate development. We generated transgenic mice expressing a constitutively active form of BmprIa (caBmprIa) in cranial neural crest (CNC) cells that contributes to the dental and palatal mesenchyme. Mice bearing enhanced BMPRIa-mediated signaling in CNC cells exhibit complete cleft palate and delayed odontogenic differentiation. We showed that the cleft palate defect in the transgenic animals is attributed to an altered cell proliferation rate in the anterior palatal mesenchyme and to the delayed palatal elevation in the posterior portion associated with ectopic cartilage formation. Despite enhanced activity of BMP signaling in the dental mesenchyme, tooth development and patterning in transgenic mice appeared normal except delayed odontogenic differentiation. These data support the hypothesis that a finely tuned level of BMPRIa-mediated signaling is essential for normal palate and tooth development.
Conflict of interest statement
Figures




Similar articles
-
BmprIa is required in mesenchymal tissue and has limited redundant function with BmprIb in tooth and palate development.Dev Biol. 2011 Jan 15;349(2):451-61. doi: 10.1016/j.ydbio.2010.10.023. Epub 2010 Oct 27. Dev Biol. 2011. PMID: 21034733 Free PMC article.
-
Altered BMP-Smad4 signaling causes complete cleft palate by disturbing osteogenesis in palatal mesenchyme.J Mol Histol. 2021 Feb;52(1):45-61. doi: 10.1007/s10735-020-09922-4. Epub 2020 Nov 7. J Mol Histol. 2021. PMID: 33159638
-
PDGFR-alpha signaling is critical for tooth cusp and palate morphogenesis.Dev Dyn. 2005 Jan;232(1):75-84. doi: 10.1002/dvdy.20197. Dev Dyn. 2005. PMID: 15543606
-
Common mechanisms in development and disease: BMP signaling in craniofacial development.Cytokine Growth Factor Rev. 2016 Feb;27:129-39. doi: 10.1016/j.cytogfr.2015.11.004. Epub 2015 Nov 24. Cytokine Growth Factor Rev. 2016. PMID: 26747371 Free PMC article. Review.
-
Gene regulatory network from cranial neural crest cells to osteoblast differentiation and calvarial bone development.Cell Mol Life Sci. 2022 Feb 27;79(3):158. doi: 10.1007/s00018-022-04208-2. Cell Mol Life Sci. 2022. PMID: 35220463 Free PMC article. Review.
Cited by
-
Understanding Mechanisms of GLI-Mediated Transcription during Craniofacial Development and Disease Using the Ciliopathic Mutant, talpid2.Front Physiol. 2016 Oct 17;7:468. doi: 10.3389/fphys.2016.00468. eCollection 2016. Front Physiol. 2016. PMID: 27799912 Free PMC article.
-
Increased activity of mesenchymal ALK2-BMP signaling causes posteriorly truncated microglossia and disorganization of lingual tissues.Genesis. 2020 Jan;58(1):e23337. doi: 10.1002/dvg.23337. Epub 2019 Sep 30. Genesis. 2020. PMID: 31571391 Free PMC article.
-
Isolation and Culture of Cranial Neural Crest Cells from the First Branchial Arch of Mice.Bio Protoc. 2022 Apr 5;12(7):e4371. doi: 10.21769/BioProtoc.4371. eCollection 2022 Apr 5. Bio Protoc. 2022. PMID: 35530521 Free PMC article.
-
Multiple tissue-specific requirements for the BMP antagonist Noggin in development of the mammalian craniofacial skeleton.Dev Biol. 2014 Aug 15;392(2):168-81. doi: 10.1016/j.ydbio.2014.06.006. Epub 2014 Jun 17. Dev Biol. 2014. PMID: 24949938 Free PMC article.
-
The effects of altered BMP4 signaling in first branchial-arch-derived murine embryonic orofacial tissues.Int J Oral Sci. 2021 Nov 29;13(1):40. doi: 10.1038/s41368-021-00142-4. Int J Oral Sci. 2021. PMID: 34845186 Free PMC article.
References
-
- Baur ST, Mai JJ, Dymecki SM (2000) Combinatorial signaling through BMP receptor IB and GDF5: shaping of the distal mouse limb and the genetics of distal limb diversity. Development 127: 605–619. - PubMed
-
- Yi SE, Daluiski A, Pederson R, Rosen V, Lyons KM (2000) The type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development 127: 621–630. - PubMed
-
- Mishina Y, Suzuki A, Ueno N, Behringer RR (1995) Bmpr encodes a type I bone morphgenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev 9: 3027–3037. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

