A novel strategy to increase the proliferative potential of adult human β-cells while maintaining their differentiated phenotype
- PMID: 23776620
- PMCID: PMC3680388
- DOI: 10.1371/journal.pone.0066131
A novel strategy to increase the proliferative potential of adult human β-cells while maintaining their differentiated phenotype
Abstract
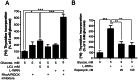
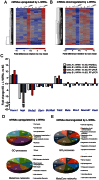
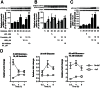
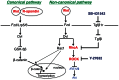
Our previous studies demonstrated that Wnt/GSK-3/β-catenin and mTOR signaling are necessary to stimulate proliferative processes in adult human β-cells. Direct inhibition of GSK-3, that engages Wnt signaling downstream of the Wnt receptor, increases β-catenin nuclear translocation and β-cell proliferation but results in lower insulin content. Our current goal was to engage canonical and non-canonical Wnt signaling at the receptor level to significantly increase human β-cell proliferation while maintaining a β-cell phenotype in intact islets. We adopted a system that utilized conditioned medium from L cells that expressed Wnt3a, R-spondin-3 and Noggin (L-WRN conditioned medium). In addition we used a ROCK inhibitor (Y-27632) and SB-431542 (that results in RhoA inhibition) in these cultures. Treatment of intact human islets with L-WRN conditioned medium plus inhibitors significantly increased DNA synthesis ∼6 fold in a rapamycin-sensitive manner. Moreover, this treatment strikingly increased human β-cell proliferation ∼20 fold above glucose alone. Only the combination of L-WRN conditioned medium with RhoA/ROCK inhibitors resulted in substantial proliferation. Transcriptome-wide gene expression profiling demonstrated that L-WRN medium provoked robust changes in several signaling families, including enhanced β-catenin-mediated and β-cell-specific gene expression. This treatment also increased expression of Nr4a2 and Irs2 and resulted in phosphorylation of Akt. Importantly, glucose-stimulated insulin secretion and content were not downregulated by L-WRN medium treatment. Our data demonstrate that engaging Wnt signaling at the receptor level by this method leads to necessary crosstalk between multiple signaling pathways including activation of Akt, mTOR, Wnt/β-catenin, PKA/CREB, and inhibition of RhoA/ROCK that substantially increase human β-cell proliferation while maintaining the β-cell phenotype.
Conflict of interest statement
Figures





Comment in
-
Diabetes: Human β-cell proliferation by promoting Wnt signalling.Nat Rev Endocrinol. 2013 Sep;9(9):502. doi: 10.1038/nrendo.2013.130. Epub 2013 Jul 2. Nat Rev Endocrinol. 2013. PMID: 23817289 No abstract available.
References
-
- Dor Y, Brown J, Martinez OI, Melton DA (2004) Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429: 41–46. - PubMed
-
- Teta M, Rankin MM, Long SY, Stein GM, Kushner JA (2007) Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 12: 817–826. - PubMed
-
- Nielsen JH, Brunstedt J, Andersson A, Frimodt-Moller C (1979) Preservation of beta cell function in adult human pancreatic islets for several months in vitro . Diabetologia 16: 97–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

