Advanced glycation end products induce peroxisome proliferator-activated receptor γ down-regulation-related inflammatory signals in human chondrocytes via Toll-like receptor-4 and receptor for advanced glycation end products
- PMID: 23776688
- PMCID: PMC3680452
- DOI: 10.1371/journal.pone.0066611
Advanced glycation end products induce peroxisome proliferator-activated receptor γ down-regulation-related inflammatory signals in human chondrocytes via Toll-like receptor-4 and receptor for advanced glycation end products
Retraction in
-
Retraction: Advanced Glycation End Products Induce Peroxisome Proliferator-Activated Receptor γ Down-Regulation-Related Inflammatory Signals in Human Chondrocytes via Toll-Like Receptor-4 and Receptor for Advanced Glycation End Products.PLoS One. 2024 May 1;19(5):e0303122. doi: 10.1371/journal.pone.0303122. eCollection 2024. PLoS One. 2024. PMID: 38691543 Free PMC article. No abstract available.
Abstract
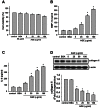
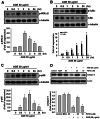
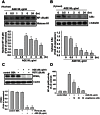
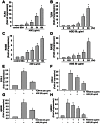
Accumulation of advanced glycation end products (AGEs) in joints is important in the development of cartilage destruction and damage in age-related osteoarthritis (OA). The aim of this study was to investigate the roles of peroxisome proliferator-activated receptor γ (PPARγ), toll-like receptor 4 (TLR4), and receptor for AGEs (RAGE) in AGEs-induced inflammatory signalings in human OA chondrocytes. Human articular chondrocytes were isolated and cultured. The productions of metalloproteinase-13 and interleukin-6 were quantified using the specific ELISA kits. The expressions of related signaling proteins were determined by Western blotting. Our results showed that AGEs enhanced the productions of interleukin-6 and metalloproteinase-13 and the expressions of cyclooxygenase-2 and high-mobility group protein B1 and resulted in the reduction of collagen II expression in human OA chondrocytes. AGEs could also activate nuclear factor (NF)-κB activation. Stimulation of human OA chondrocytes with AGEs significantly induced the up-regulation of TLR4 and RAGE expressions and the down-regulation of PPARγ expression in a time- and concentration-dependent manner. Neutralizing antibodies of TLR4 and RAGE effectively reversed the AGEs-induced inflammatory signalings and PPARγ down-regulation. PPARγ agonist pioglitazone could also reverse the AGEs-increased inflammatory signalings. Specific inhibitors for p38 mitogen-activated protein kinases, c-Jun N-terminal kinase and NF-κB suppressed AGEs-induced PPARγ down-regulation and reduction of collagen II expression. Taken together, these findings suggest that AGEs induce PPARγ down-regulation-mediated inflammatory signalings and reduction of collagen II expression in human OA chondrocytes via TLR4 and RAGE, which may play a crucial role in the development of osteoarthritis pathogenesis induced by AGEs accumulation.
Conflict of interest statement
Figures







Similar articles
-
The Role of PPARγ in Advanced Glycation End Products-Induced Inflammatory Response in Human Chondrocytes.PLoS One. 2015 May 29;10(5):e0125776. doi: 10.1371/journal.pone.0125776. eCollection 2015. PLoS One. 2015. PMID: 26024533 Free PMC article.
-
Advanced glycation end products induce the expression of interleukin-6 and interleukin-8 by receptor for advanced glycation end product-mediated activation of mitogen-activated protein kinases and nuclear factor-κB in human osteoarthritis chondrocytes.Rheumatology (Oxford). 2011 May;50(5):838-51. doi: 10.1093/rheumatology/keq380. Epub 2010 Dec 20. Rheumatology (Oxford). 2011. PMID: 21172926 Free PMC article.
-
Advanced glycation end products downregulates peroxisome proliferator-activated receptor γ expression in cultured rabbit chondrocyte through MAPK pathway.Eur J Pharmacol. 2010 Dec 15;649(1-3):108-14. doi: 10.1016/j.ejphar.2010.09.025. Epub 2010 Sep 20. Eur J Pharmacol. 2010. PMID: 20863825
-
Advanced glycation endproducts and osteoarthritis.Curr Rheumatol Rep. 2003 Feb;5(1):33-40. doi: 10.1007/s11926-003-0081-x. Curr Rheumatol Rep. 2003. PMID: 12590883 Review.
-
The dynamic roles of advanced glycation end products.Vitam Horm. 2024;125:1-29. doi: 10.1016/bs.vh.2024.02.008. Epub 2024 May 28. Vitam Horm. 2024. PMID: 38997161 Review.
Cited by
-
Advanced glycation end-products induce apoptosis in pancreatic islet endothelial cells via NF-κB-activated cyclooxygenase-2/prostaglandin E2 up-regulation.PLoS One. 2015 Apr 21;10(4):e0124418. doi: 10.1371/journal.pone.0124418. eCollection 2015. PLoS One. 2015. PMID: 25898207 Free PMC article.
-
Activation of GPR40 Suppresses AGE-Induced Reduction of Type II Collagen and Aggrecan in Human SW1353 Chondrocytes.Drug Des Devel Ther. 2020 Jun 15;14:2371-2379. doi: 10.2147/DDDT.S239273. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32606604 Free PMC article.
-
The Protective Effect of Brazilian Propolis against Glycation Stress in Mouse Skeletal Muscle.Foods. 2019 Sep 25;8(10):439. doi: 10.3390/foods8100439. Foods. 2019. PMID: 31557885 Free PMC article.
-
Dietary Advanced Glycation End Products and Their Potential Role in Cardiometabolic Disease in Children.Horm Res Paediatr. 2016;85(5):291-300. doi: 10.1159/000444053. Epub 2016 Mar 19. Horm Res Paediatr. 2016. PMID: 27008270 Free PMC article. Review.
-
ATP synthase subunit-β down-regulation aggravates diabetic nephropathy.Sci Rep. 2015 Oct 9;5:14561. doi: 10.1038/srep14561. Sci Rep. 2015. PMID: 26449648 Free PMC article.
References
-
- Glass GG (2006) Osteoarthritis. Dis Mon 52: 343–362. - PubMed
-
- Goldring MB, Goldring SR (2007) Osteoarthritis. J Cell Physiol 213: 626–634. - PubMed
-
- Blagojevic M, Jinks C, Jeffery A, Jordan KP (2010) Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage 18: 24–33. - PubMed
-
- Felson DT, Zhang Y (1998) An update on the epidemiology of knee and hiposteoarthritis with a view to prevention. Arthritis Rheum 41: 1343–1355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

