Cutaneous adverse drug reactions in a tertiary care teaching hospital: A North Indian perspective
- PMID: 23776774
- PMCID: PMC3657944
- DOI: 10.4103/2229-516X.81982
Cutaneous adverse drug reactions in a tertiary care teaching hospital: A North Indian perspective
Abstract
Background: Cutaneous manifestations of adverse drug reactions are a common occurrence and need to be differentiated from other causes of similar manifestations. Active search is essential for identification of these as patients may tend to downplay the causal association between drug use and the subsequent cutaneous manifestation.
Purpose: To study the incidence of Cutaneous Adverse Drug Reactions (CADRs) in a tertiary care teaching hospital in North India.
Methods: A prospective, observational study was conducted over a period of 6 months; using self-reporting method for selection of cases. The CADRs were graded as definite, possible and probable.
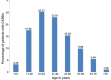
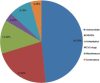
Results: During the study period, 91 cases of CADRs were observed. Maximum incidence of CADRs was seen with antimicrobials (48.30%), followed by nonsteroidal anti-inflammatory drugs (21.90%). Maculopapular rash was the most common cutaneous manifestation of ADRs (42.85%).
Conclusion: CADRs are a common occurrence and awareness about the same is essential for diagnosis and prevention.
Keywords: Cutaneous adverse drug reactions; drug rash; drug reaction; pharmacovigilance.
Conflict of interest statement
Figures
References
-
- Nerurkar RP, Nadkar MY, Bichile SK. Need for monitoring adverse drug reactions. J Assoc Physicians India. 1998;46:673–4. - PubMed
-
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–5. - PubMed
-
- Ramesh M, Pandit J, Parthasarathi G. Adverse drug reactions in a south Indian hospital--their severity and cost involved. Pharmacoepidemiol Drug Saf. 2003;12:687–92. - PubMed
-
- Bigby M. Rates of cutaneous reactions to drugs. Arch Dermatol. 2001;137:765–70. - PubMed
-
- Pudukadan D, Thappa DM. Adverse cutaneous drug reactions: clinical pattern and causative agents in a tertiary care center in South India. Indian J Dermatol Venereol Leprol. 2004;70:20–4. - PubMed