Conjugation site heterogeneity causes variable electrostatic properties in Fc conjugates
- PMID: 23777335
- PMCID: PMC3713463
- DOI: 10.1021/bc4000564
Conjugation site heterogeneity causes variable electrostatic properties in Fc conjugates
Abstract
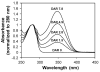
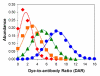
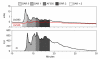
Immunoconjugates, including antibody-drug conjugates and Fc-conjugates, represent a rapidly growing class of therapeutics undergoing clinical development. Despite their growing popularity, the high intrinsic heterogeneity of immunoconjugates often complicates the development process and limits their widespread application. In particular, immunoconjugate charge variants exhibit markedly different colloidal stabilities, solubilities, pharmacokinetics, and tissue distributions. Charge variants arise spontaneously due to degradation and, depending on the type of drug, linker, and conjugation site, through drug conjugation. Electrostatic changes in naked antibodies often result in poor performance characteristics, and therefore, charge alterations due to degradation are critical to control. Charge properties are expected to be equally important to producing well-behaved ADCs. Charge-based methods of analysis, such as isoelectric focusing and ion exchange chromatography, are capable of probing the underlying complexities within immunoconjugate drug products. Despite the utility of these methods, there are only a few published reports of charge-based assays applied to immunoconjugates. In the present study, we sought to identify the effects of chemical conjugation on the electrostatic properties of Fc-conjugates. In order to minimize the effects of post-translational modifications (e.g., deamidation), a single Fc charge variant was isolated prior to conjugation of a fluorescent probe, Alexa Fluor 350, to the side chains of lysine residues. The resulting Fc-conjugates were assessed by a variety of analytical techniques, including isoelectric focusing and ion exchange chromatography, to determine their charge properties.
Figures


References
-
- Sapra P, Hooper AT, O’Donnell CJ, Gerber H-P. Investigational antibody drug conjugates for solid tumors. Expert Opin. Invest. Drugs. 2011;20:1131–1149. - PubMed
-
- Boswell CA, Mundo EE, Zhang C, Bumbaca D, Valle NR, Kozak KR, Fourie A, Chuh J, Koppada N, Saad O, Gill H, Shen B-Q, Rubinfeld B, Tibbitts J, Kaur S, Theil F-P, Fielder PJ, Khawli LA, Lin K. Impact of Drug Conjugation on Pharmacokinetics and Tissue Distribution of Anti-STEAP1 Antibody–Drug Conjugates in Rats. Bioconjugate Chem. 2011;22:1994–2004. - PubMed
-
- Wakankar AA, Feeney MB, Rivera J, Chen Y, Kim M, Sharma VK, Wang YJ. Physicochemical stability of the antibody-drug conjugate Trastuzumab-DM1: changes due to modification and conjugation processes. Bioconjugate Chem. 2010;21:1588–95. - PubMed
-
- Vlasak J, Ionescu R. Heterogeneity of monoclonal antibodies revealed by charge-sensitive methods. Curr. Pharm. Biotechnol. 2008;9:468–81. - PubMed
-
- Khawli LA, Goswami S, Hutchinson R, Kwong ZW, Yang J, Wang X, Yao Z, Sreedhara A, Cano T, Tesar DB, Nijem I, Allison DE, Wong PY, Kao Y-H, Quan C, Joshi A, Harris RJ, Motchnik P. Charge variants in IgG1: Isolation, characterization, in vitro binding properties and pharmacokinetics in rats. MAbs. 2010;2:613–624. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

